A facile protein purification method for localizing recombinant proteins to the cell surface
A recombinant protein and cell surface technology, applied in the field of simple purification of protein, to save time and cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Construction and expression of Lpp-OmpA-(SNAC-tag)-GB1 fusion protein and Lpp-OmpA-(SNAC-tag)-SFGFP fusion protein
[0045] The mode of the fusion protein used in this embodiment is as follows figure 1 As shown, the code Lpp(1-9)-OmpA(45-159)-(SNAC-tag)-GB1 (sequence shown in SEQ ID NO.1) obtained by gene synthesis (Sangon Bioengineering Co., Ltd.) Shown) the pet30a plasmid was transformed into E.coli BL21(DE3), the expression was induced by 0.5mM IPTG at 30°C for 5h, and the bacteria were harvested by a high-speed centrifuge. and stored at -80°C until use. The pet30a plasmid encoding Lpp(1-9)-OmpA(45-159)-(SNAC-tag)-SFGFP (sequence shown in SEQ ID NO.2) was obtained by total gene synthesis (Sangon Bioengineering Co., Ltd.) Express in the same way and collect bacteria.
Embodiment 2
[0046] Embodiment 2: Construction, expression and purification of 6His-GB1 fusion protein and 6His-SFGFP fusion protein
[0047] The pet28a plasmid encoding 6His-GB1 (sequence shown in SEQ ID NO.3) obtained by gene synthesis (Sangon Bioengineering Co., Ltd.) was transformed into E.coli BL21 (DE3), using 0.5mM IPTG in The expression was induced at 30°C for 5 hours, and the bacteria were harvested using a high-speed centrifuge. and stored at -80°C until use. The pet28a plasmid encoding 6His-SFGFP (sequence shown in SEQ ID NO.4) was obtained by total gene synthesis (Sangon Bioengineering Co., Ltd.) and obtained and expressed in the same manner, and the bacteria were harvested.
Embodiment 3
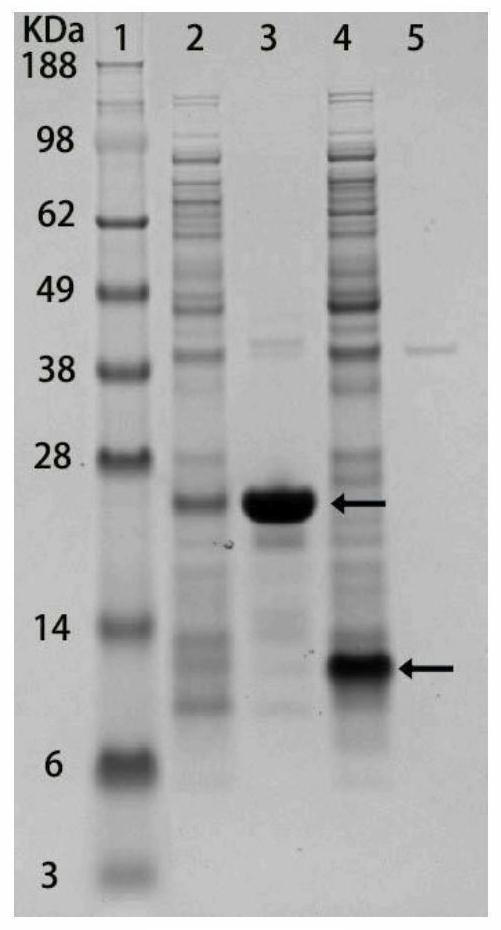
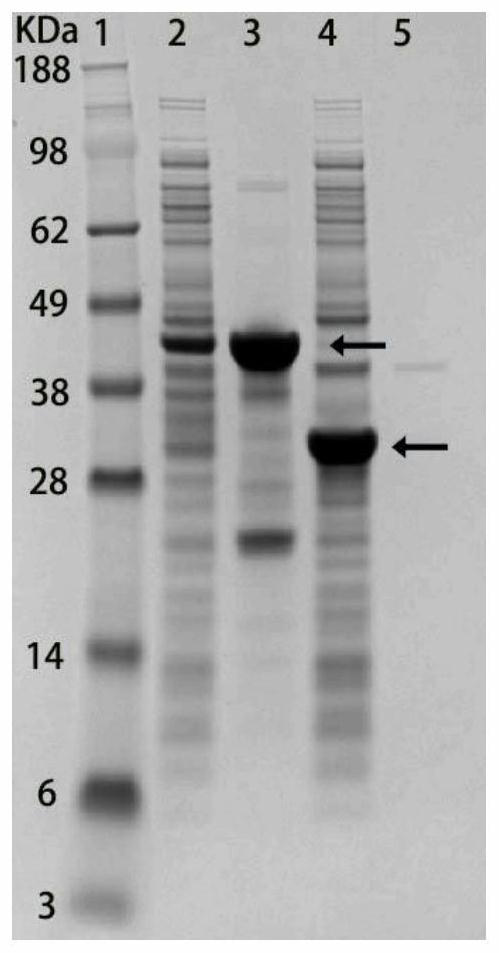
[0048] Example 3: Identification of cell membrane and cytoplasmic components of bacteria expressing fusion protein
[0049] In order to characterize whether the signal peptide can localize the target protein to the cell membrane, it is necessary to identify the cell membrane and cytoplasmic components of the bacteria expressing the recombinant protein by SDS-PAGE gel. Bacteria were resuspended in 10 mM PBS (pH 7.4) and lysed using sonication. The supernatant and the precipitate were separated by high-speed centrifugation at 12000 rpm, and the precipitate was eluted twice with TDSET buffer (1% Triton X-100, 0.2% sodium deoxycholate, 0.1% SDS, 10 mM tetrasodium EDTA, and 10 mM Tris / HCl). Pellets and supernatants were identified using SDS-PAGE. figure 2 The 2nd lane is the supernatant of bacteria expressing Lpp-OmpA-(SNAC-tag)-GB1, the 3rd lane is the cell membrane components of Lpp-OmpA-(SNAC-tag)-GB1 bacteria, and the 4th lane is expressing 6His- The supernatant of GB1 bacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com