Human respiratory tract epithelial cell pathogen cytology detection method and kit
A technology of epithelial cells and detection methods, applied in measuring devices, scientific instruments, instruments, etc., can solve problems such as large differences in viruses, undisclosed antigen and antibody detection methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] This embodiment provides a general cytological detection method for human upper respiratory tract epithelial cell pathogens, comprising the following steps:
[0095] S1. Specimen collection and preparation:
[0096] Collect upper respiratory tract specimens such as nasopharynx, oral epithelial cells or sputum, put them in the cell preservation solution in the sampling tube and fix them, and then prepare sample slides. The methods include but are not limited to the following two:
[0097] a. Cell manual smear: collect the cells in the sampling tube for cell smear and cell fixation;
[0098] b. Automatic cell smear: automatic liquid-based thin-layer cell film maker to prepare sample slides and fix cells;
[0099] S2, ICC immunocytochemical staining technique (abbreviation, antibody detection)
[0100] Methods include but are not limited to the following two, as follows:
[0101] a. Instrument testing:
[0102] Put the reagents and prepared samples into the automatic imm...
Embodiment 2
[0134] The influence of embodiment two cell preservation solution on fixation effect
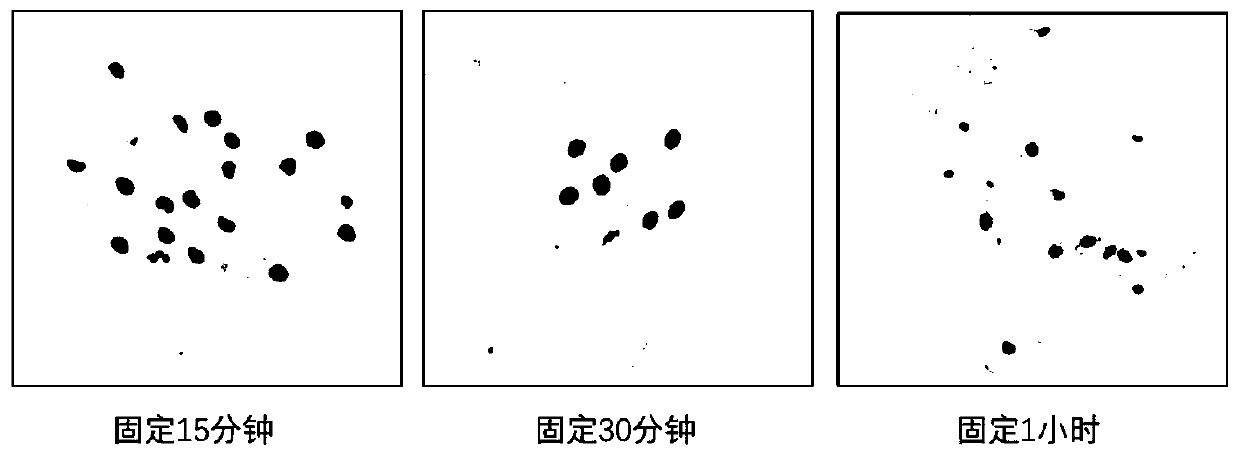
[0135] It should be noted that different cell preservation solutions have a greater impact on the fixation effect of the sample. This example provides comparative test data on the influence of different cell preservation solutions on the fixation effect, as follows:
[0136] 1. The effect of cell preservation solution on the number of cells
[0137] Take 6x10 7 Remove the supernatant of the 293T cells, add PBS to resuspend, divide into six parts on average, as samples 1, 2, 3, 4, 5, and 6, repeat at least 3 times for each sample, and calculate the average value of the experimental results. Centrifuge again, remove the supernatant, and add samples 1, 2, 3, 4, 5, and 6 respectively:
[0138] Sample 1: 1% mass fraction of paraformaldehyde, 75% volume fraction of ethanol
[0139] Sample 2: 1% mass fraction of paraformaldehyde, 60% volume fraction of ethanol
[0140] Sample 3: 1% mass fractio...
Embodiment 3
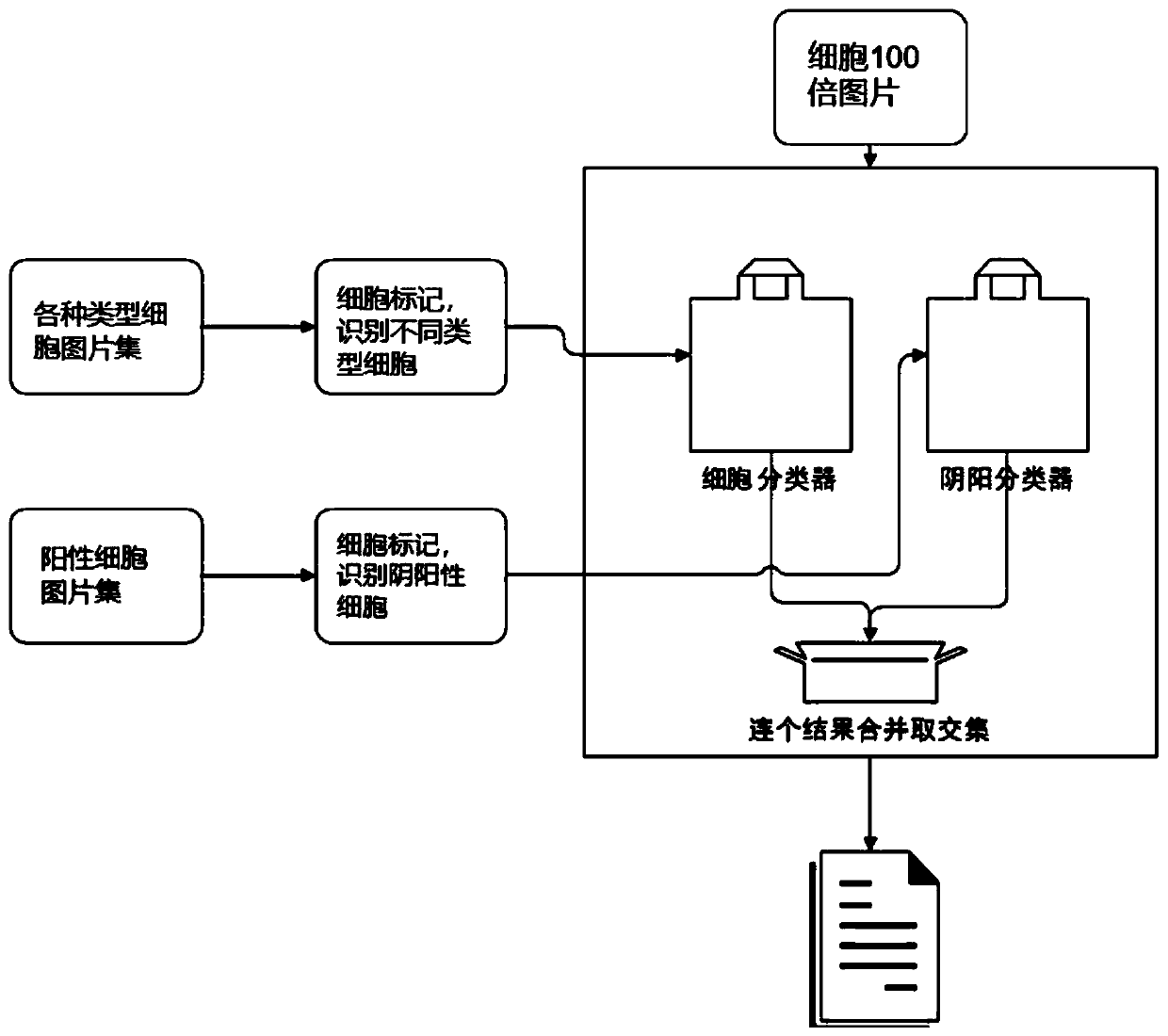
[0174] Embodiment 3 The steps and logic of computer automatic judgment positive
[0175] This embodiment provides a method based on computer artificial intelligence technology to automatically count pictures in the server and identify positive cells, and output an auxiliary diagnosis report, as follows:
[0176] S1. Collect the types of pictures that need to be interpreted, including negative and positive, and sample pictures from different parts, and perform cell labeling.
[0177] S2. Carry out model training and evaluate the accuracy of the model. Review the data annotation, continue training, and optimize the algorithm.
[0178] S3. When the accuracy of the model meets expectations, the test can be performed.
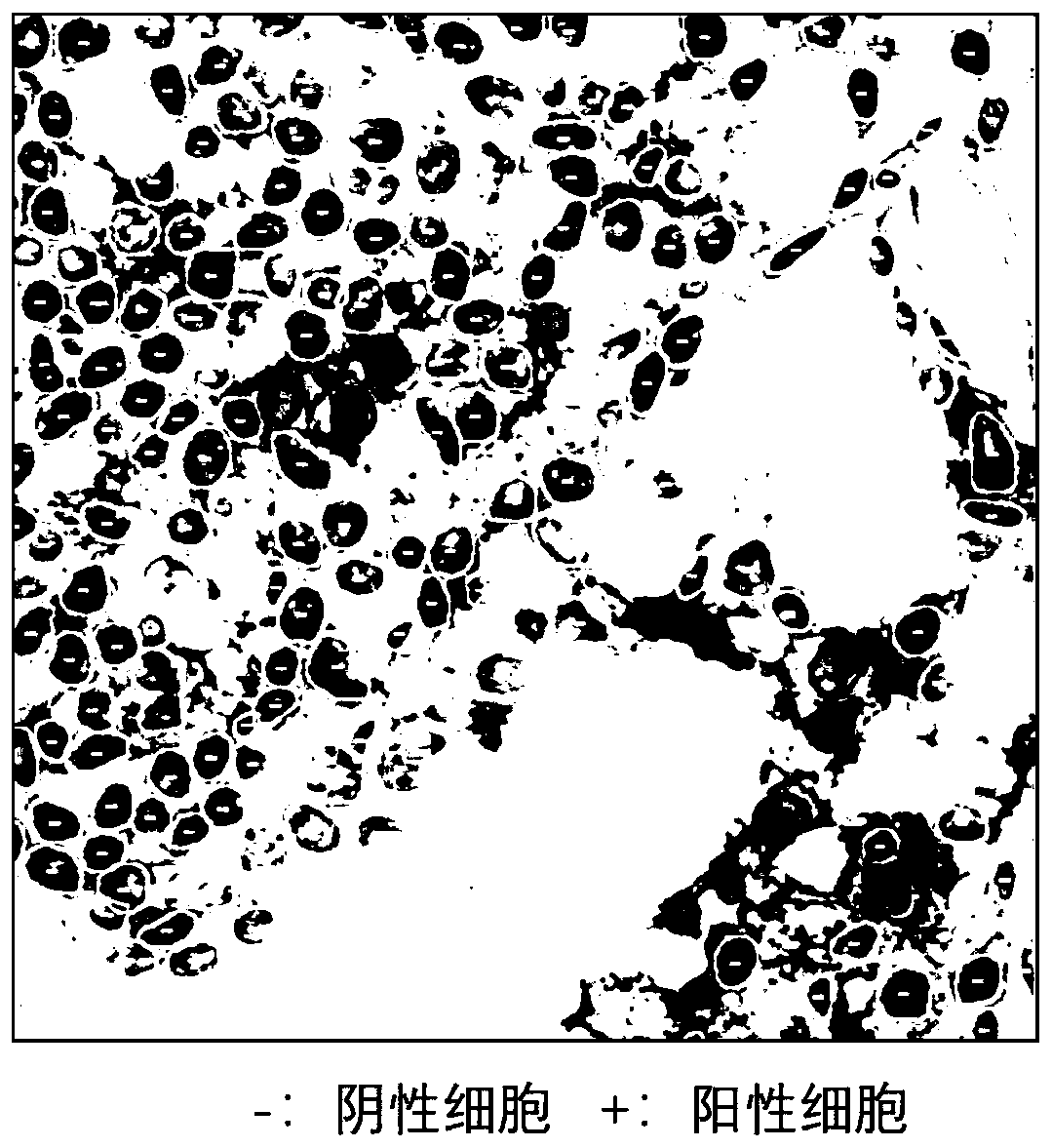
[0179] See specific steps figure 2 , see the output picture result image 3 . Since the computer automatic judgment technology is not the key point of the present invention, it will not be described in detail here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com