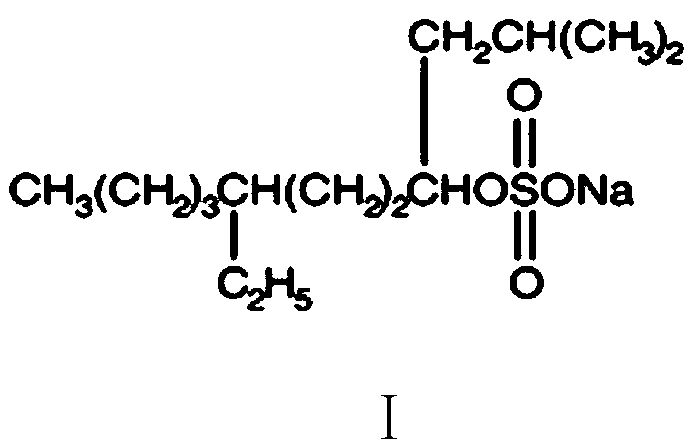
Application of sodium tetradecyl sulfate in preparation of medicine for treating hemangioma
A technology of sodium tetradecyl sulfate and hemangioma, applied in the direction of drug combination, antineoplastic drugs, active ingredients of anhydride/acid/halide, etc., can solve side effects and other problems, achieve good curative effect and avoid huge trauma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Isolation and cultivation of hemangioma cells
[0046] Place the surgically resected hemangioma tissue in serum-free DMEM culture medium, and rinse the blood clot and necrotic tissue of the hemangioma specimen with PBS until the washing solution is clear in color without turbidity and exfoliated tissue; use ophthalmic scissors Use tweezers to remove excess skin and fat, select tumor tissue, and trim it to 1-2cm 3 Add 0.2% type Ⅰ collagenase pre-warmed at 37°C, digest in a water bath at 37°C, shake once every 5 minutes, shake vigorously after 20 minutes of digestion, then add 0.1% trypsin to digest at 37°C for 5 minutes, and finally add 20mL DMEM serum-free medium to stop the digestion, filter with a 100-mesh metal sieve to remove undigested tissue. Trim the digested tissue block to 1mm 3 Size, placed in 2ml fetal bovine serum, let stand for 5min, then suspended in endothelial cell medium (composition: M199 medium, 10% fetal bovine serum, 10U / mL penicillin, 1...
Embodiment 2
[0047] The establishment of embodiment 2 mouse model
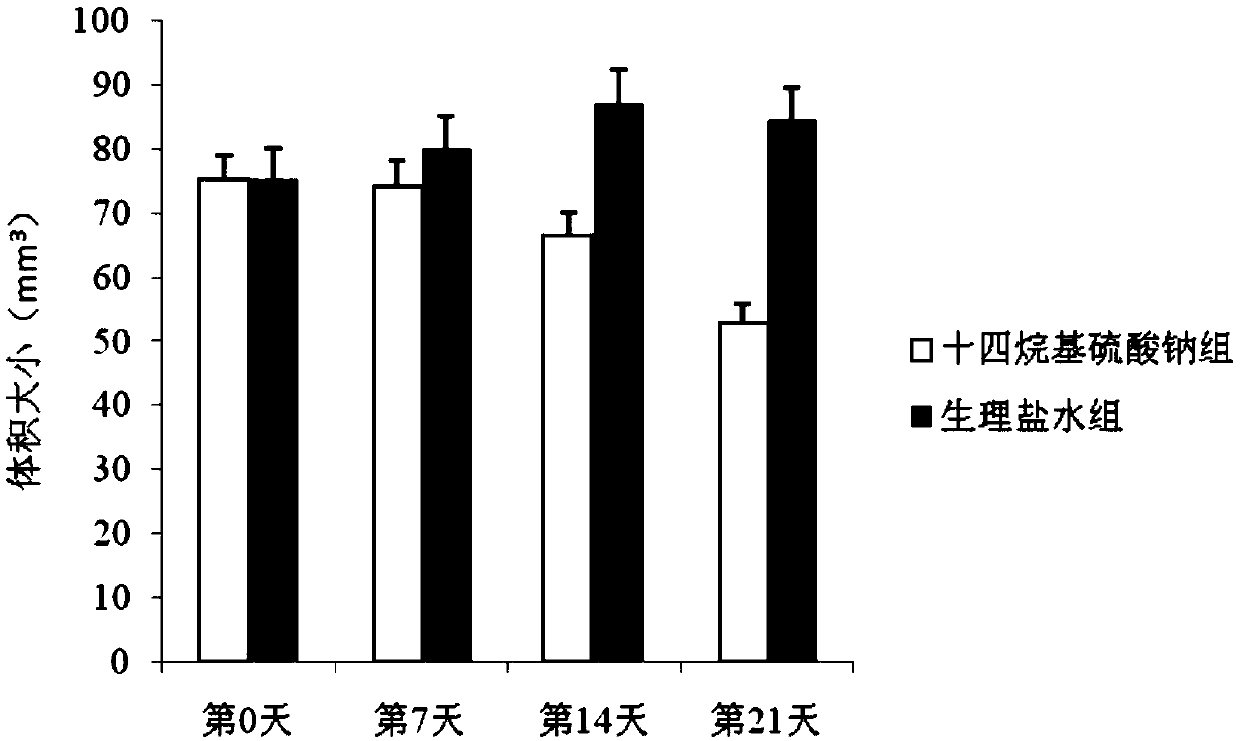
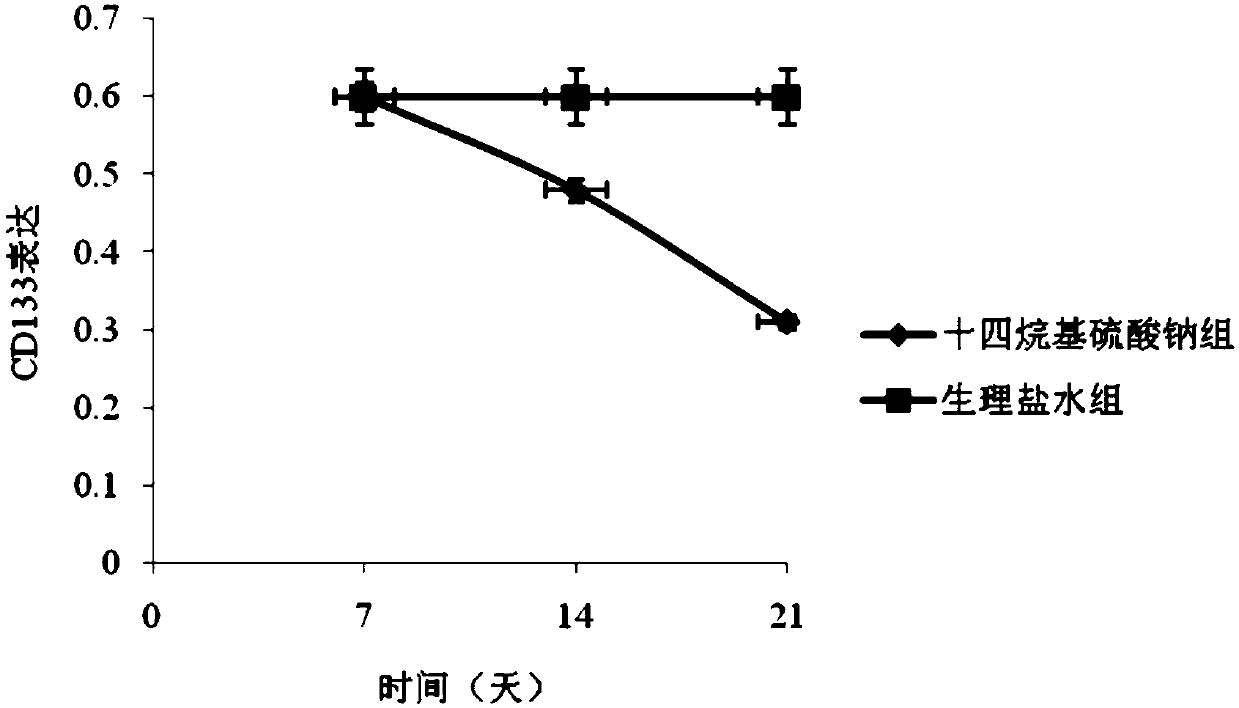
[0048] The 3rd generation hemangioma cells collected by trypsinization were digested with 10 8 The cell / mL density was resuspended in complete culture medium, and 200 μL was injected subcutaneously into the bilateral buttocks of 40 nude mice. On the 48th day after injection, 36 of the injected tumor cells survived. There was no big difference in the size of the tumors, the size was about 75mm 3 , were randomly divided into two groups, 18 in each group, marked as group A and group B respectively, group A was injected with 1mL sodium tetradecyl sulfate (2mL: 20mg), twice a day, group B was injected with 1mL normal saline As a control group, twice a day. Observe the growth of the tumor every 7 days, calculate the maximum diameter a and transverse diameter b of the tumor with a vernier caliper, and use the formula V=π / 6×a×b 2 Calculate the volume of the tumor. On the 7th day, 14th day, and 21st day after drug intervention,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com