Primer set for triple detection of Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum, product and application
A technology for Chlamydia trachomatis and Ureaplasma urealyticum, which is applied to the determination/inspection of microorganisms, microorganisms, recombinant DNA technology, etc., can solve the problems of increasing the cost of detection and cumbersomeness, and achieve fast and intuitive detection results, strong repeatability and specificity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] This embodiment provides a triple detection kit for Neisseria gonorrhoeae, Chlamydia trachomatis and Ureaplasma urealyticum, including: PCR reaction solution, primer set mixture, positive control substance and negative control substance.
[0111] Among them, the PCR reaction solution includes 10×PCR Buffer, 25mM MgCl 2 , 25mM dNTPs, 100mM dUTP, Taq enzyme and UDG enzyme.
[0112] Primer set mix includes:
[0113] Component (i): consists of a pair of primers for detecting Chlamydia trachomatis and a probe for detecting Chlamydia trachomatis, wherein the base sequences of the two primers are shown in SEQ ID NO.3 and SEQ ID NO.4 respectively, and the probe The base sequence of the probe is shown in SEQ ID No.8, the 5' end of the probe is marked with a fluorescent reporter group (FAM), and the 3' end is marked with a fluorescent quencher group (BHQ-1); wherein, the Chlamydia trachomatis primer The ratio with the probe is: Chlamydia trachomatis-F: Chlamydia trachomatis-R: ...
Embodiment 2
[0144] Embodiment 2 Positive control substance detection result
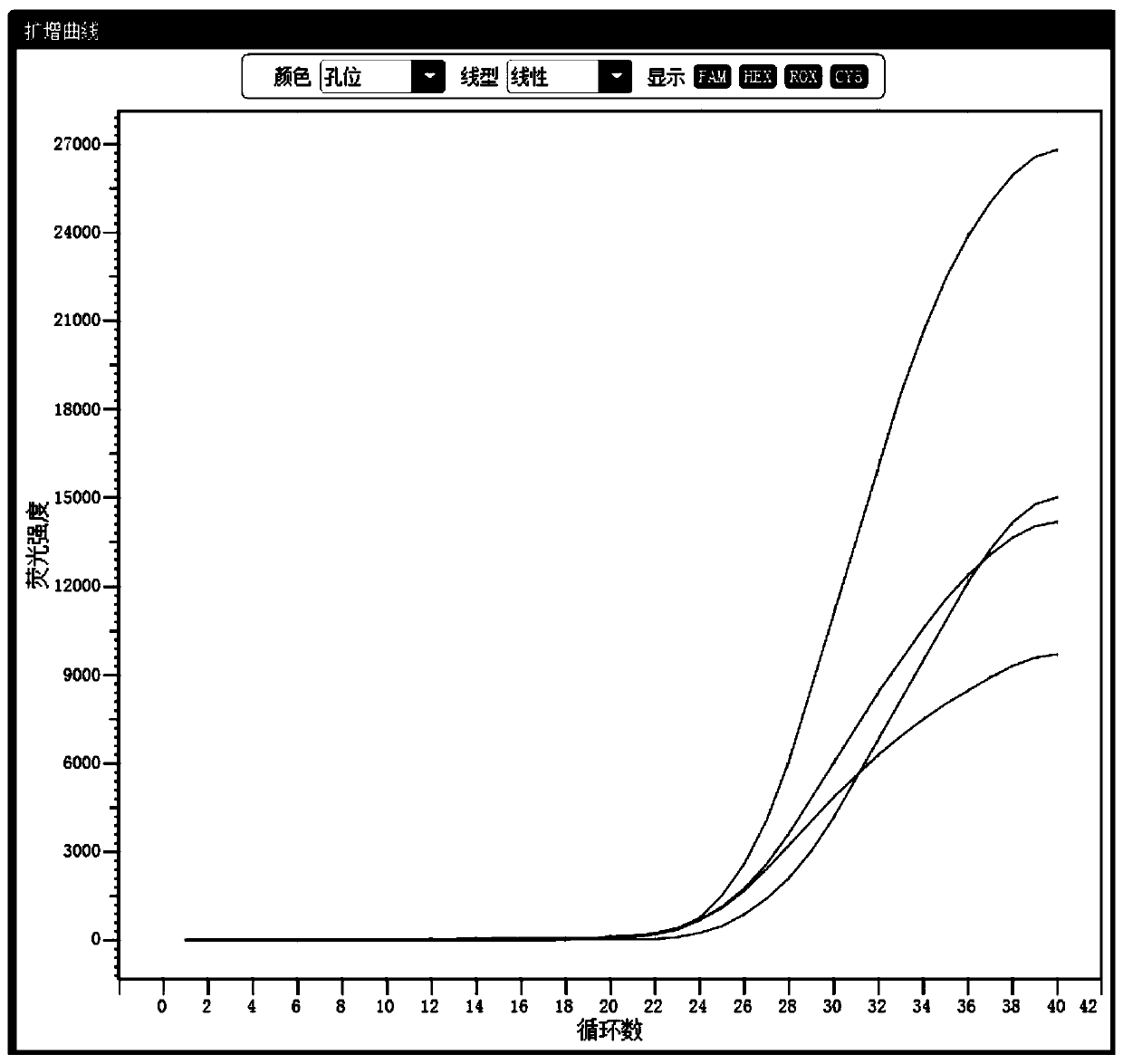
[0145] The positive control substance was detected using the kit and detection method provided in Example 1 of the present invention. The positive control substance is a mixed plasmid containing target fragments of target genes amplified by Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), and Ureaplasma urealyticum (UU). figure 1 .
Embodiment 3
[0146] Embodiment 3 sensitivity test
[0147] Adopt the kit and detection method that the embodiment of the present invention 1 provides to detect:
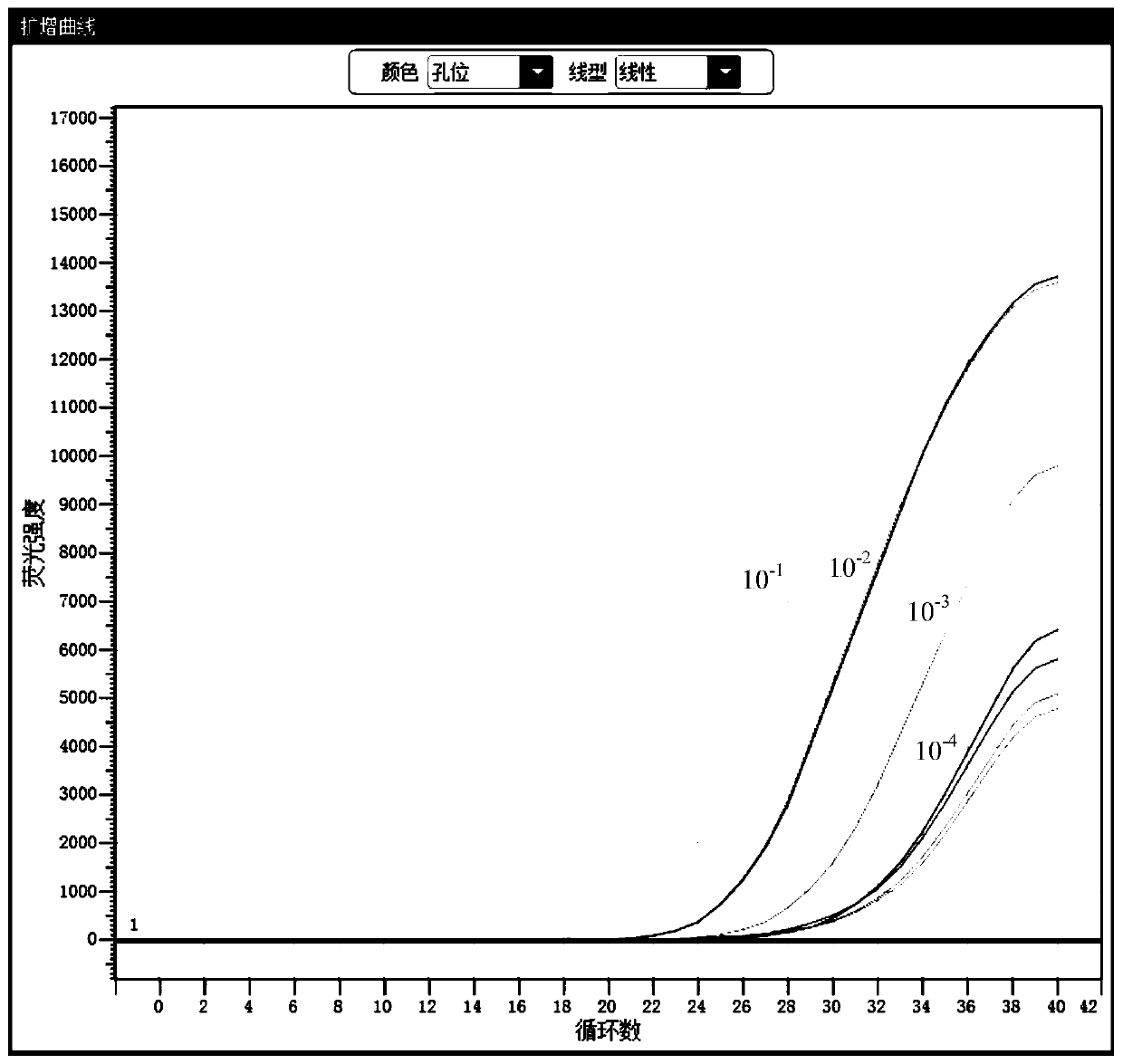
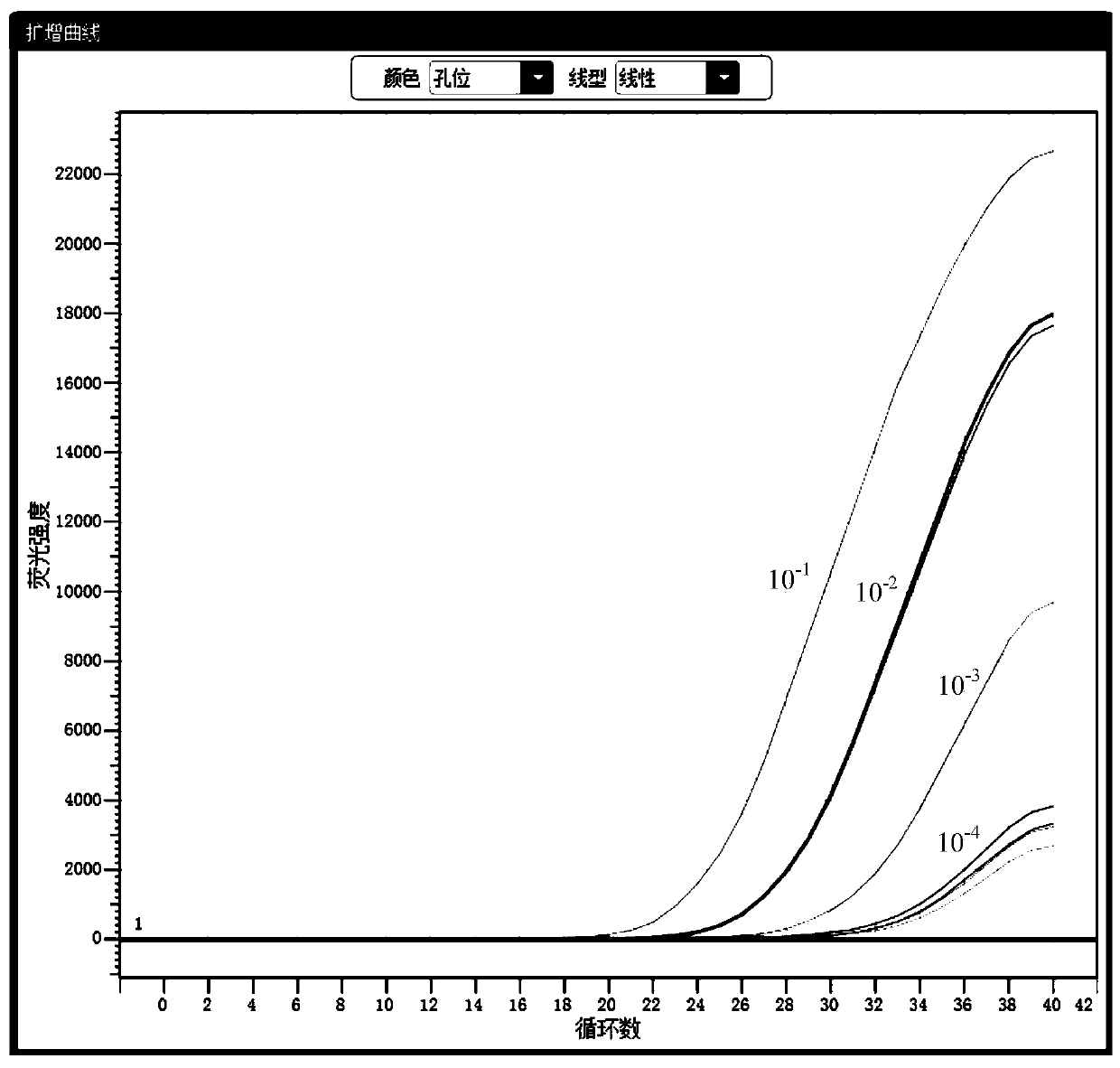
[0148] The identified clinical samples of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Ureaplasma urealyticum (UU) were diluted 10 times and 10 times respectively as initial samples. 2 times, 10 3 times, 10 4 Times, the test results show that: the kit has good sensitivity, and presents a gradient change, see the results figure 2 , image 3 , Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com