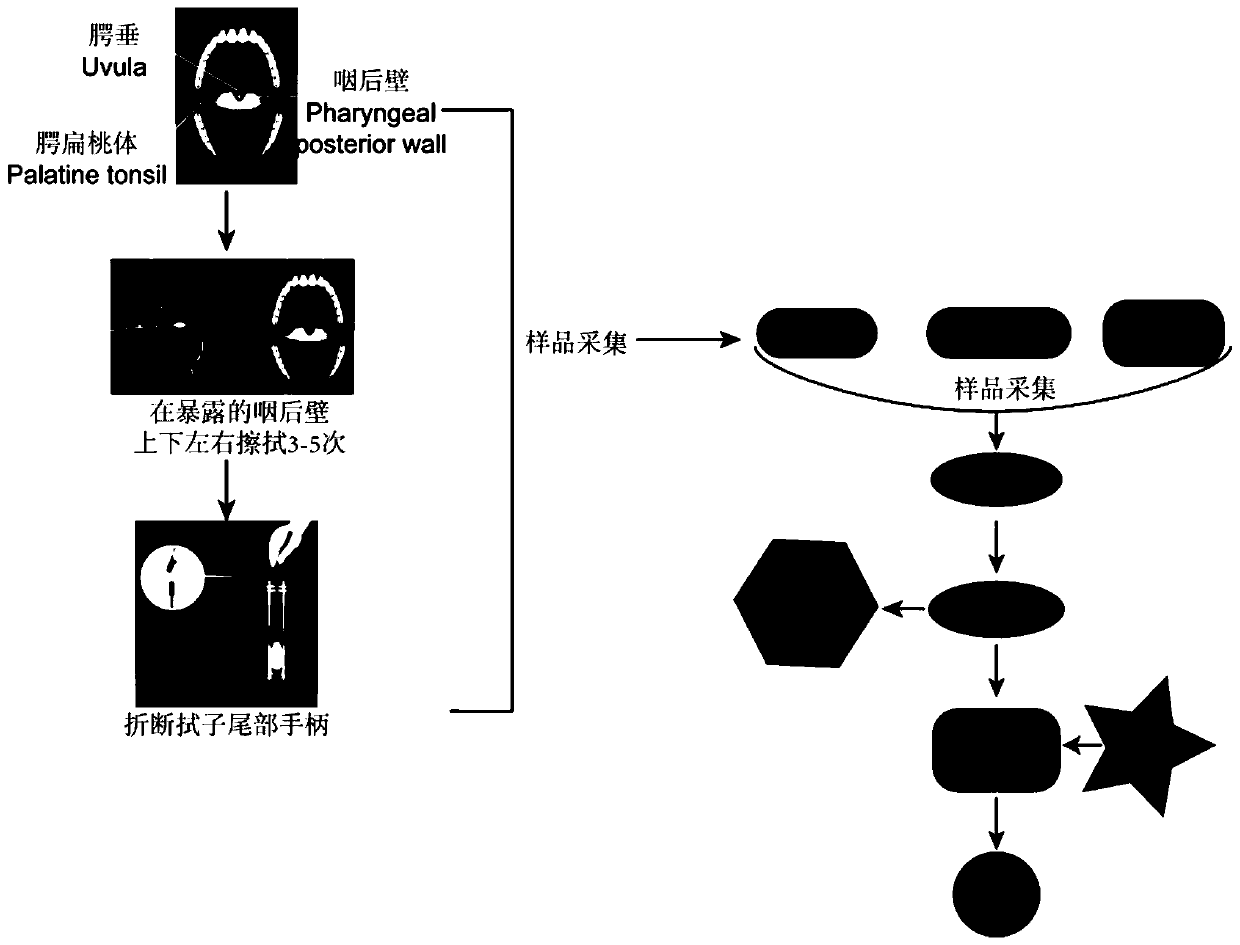
Detection kit for novel coronavirus COVID-19 infection
A technology for COVID-19 and coronavirus, applied in the field of new coronavirus COVID-19 infection detection kits, can solve the problems of increased detection costs, increased internal standard gene detection, inability to determine, etc., to ensure detection sensitivity and ensure biological safety. The effect of increasing the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
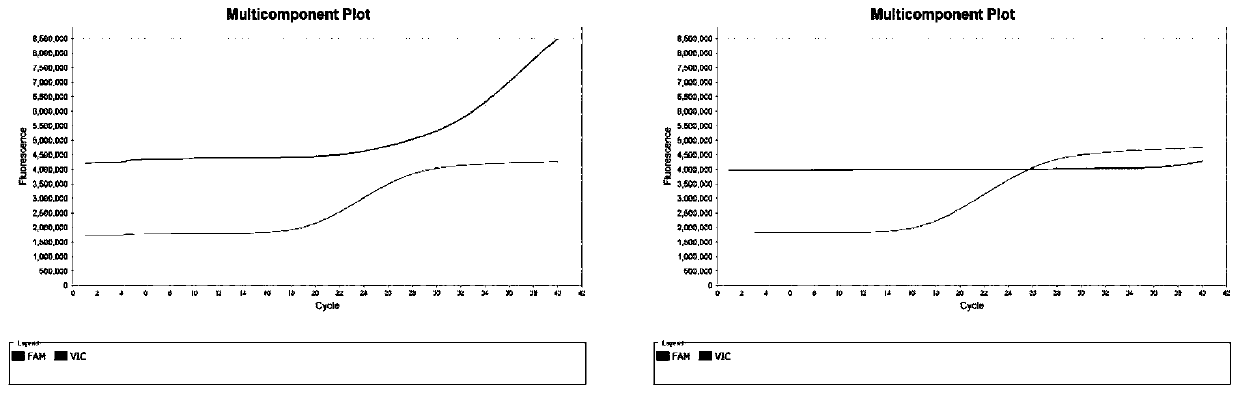
[0086] The above protocol was used to collect nasal swabs and oropharyngeal swabs from 11 individuals, as well as cerebrospinal fluid and blood samples from 8 patients, RNA extraction, and fluorescent quantitative PCR detection. The results are shown in Table 1 and figure 2 .
[0087] The Ct value of the positive internal reference gene GAPDH detection of nasal swab and oropharyngeal swab is between 12-25 (Table 5), the Ct value of the positive internal reference gene GAPDH detection of cerebrospinal fluid sample is between 23-31, and the positive internal reference gene GAPDH detection of blood sample The Ct value detected by the internal reference gene GAPDH was between 16-18 (Table 6), which met the requirements for sample preservation and extraction; The new coronavirus, which is not found in the preservation and extraction of existing methods, fully proves the reliability, stability and sensitivity of Trizol reagent for preserving samples and performing nucleic acid extr...
Embodiment 3
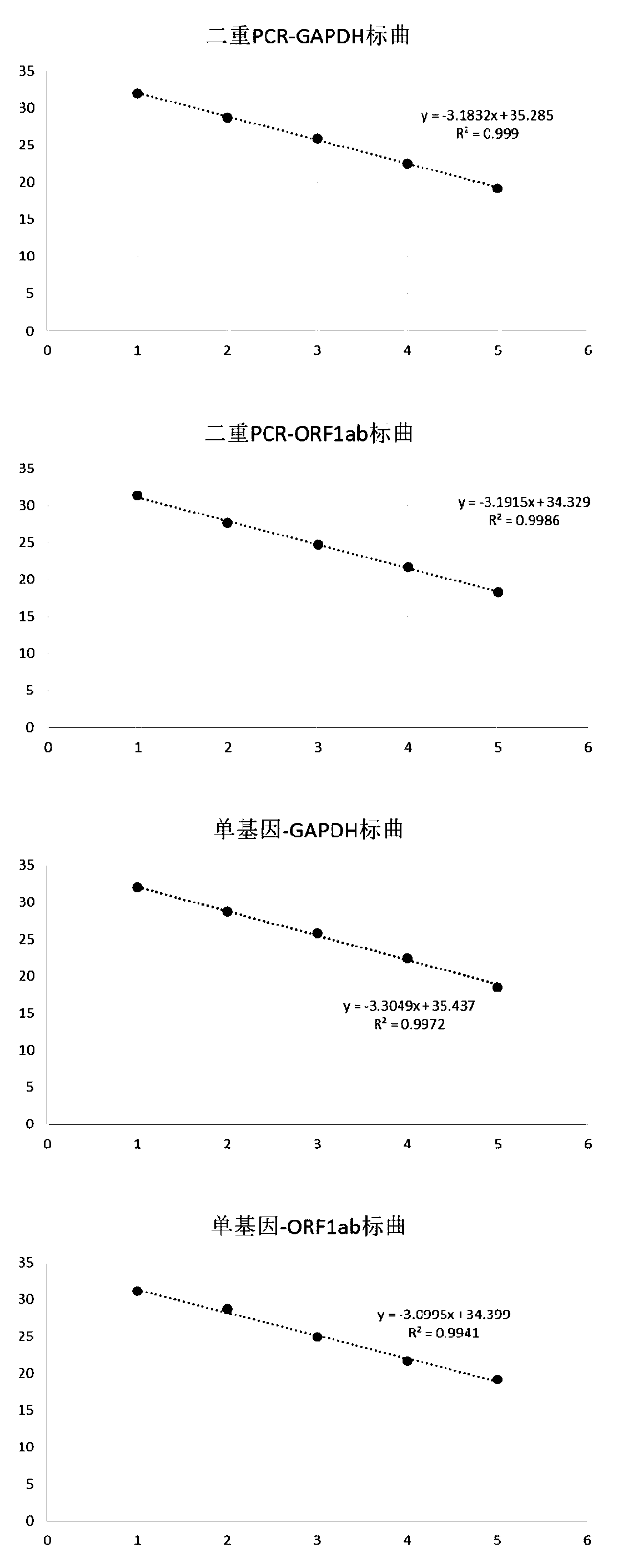
[0095] Using the above fluorescent quantitative PCR detection method to carry out single gene detection and double PCR detection on GAPDH, ORF1ab standard products, the results are shown in Table 7 and image 3. It can be seen from the results that the Ct value of the single-gene detection and the Ct value of the double PCR detection are almost the same for the standards with the same number of molecules, indicating that the specificity of the primers is better, and there is no significant difference between the two groups of primers and probes. Influence, the linear relationship is also very good.
[0096] Table 7
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com