A kind of enzymatic cationic liposome and its application
A cationized, liposome technology, applied in the directions of liposome delivery, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. The anti-cancer effect and other issues of the prime factor to achieve the effect of promoting enrichment and penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
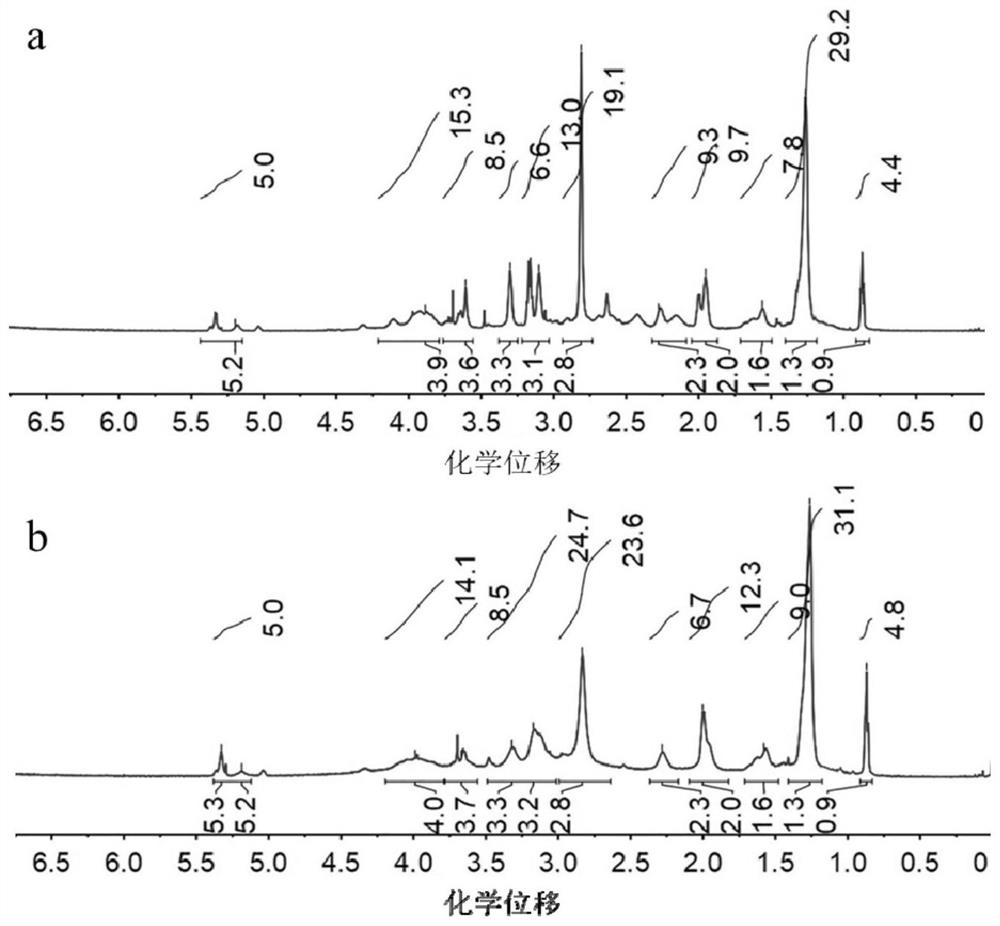
[0038] Example 1: Preparation of DOPE-GSH and contrast DOPE-EGG
[0039] Preparation of DOPE-GSH:
[0040] 1) Add Boc-protected lysine (Boc-Lys, 0.35 g, 1 mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt in dichloromethane (DCM, 10 mL) salt (EDC, 0.39g, 2mmol) and N-hydroxysuccinimide (NHS, 0.23g, 2mmol), reacted at room temperature for 4h, then added dioleoylphosphatidylethanolamine (DOPE, 0.75g, 1mmol), at room temperature Under reaction 12h.
[0041] 2) Add trifluoroacetic acid (TFA, 10 mL) and react at room temperature for 4 h. After concentrated by rotary evaporation, dialyzed in methanol (molecular weight cut-off: 500Da) for 24h, and dried by rotary evaporation, a lysine-modified DOPE product (DOPE-Lys, about 0.65g, yield 74.5%) was obtained.
[0042] 3) Add Boc-γ-Glu(otbu)-Cys(Trt)-Gly(pro-GSH, 0.71g, 1mmol), EDC (0.39g, 2mmol) and NHS (0.23g, 2mmol) in DCM (10mL) , reacted at room temperature for 4h, then added DOPE-Lys (0.38g, 0.5mmol), and reacted at room...
Embodiment 2
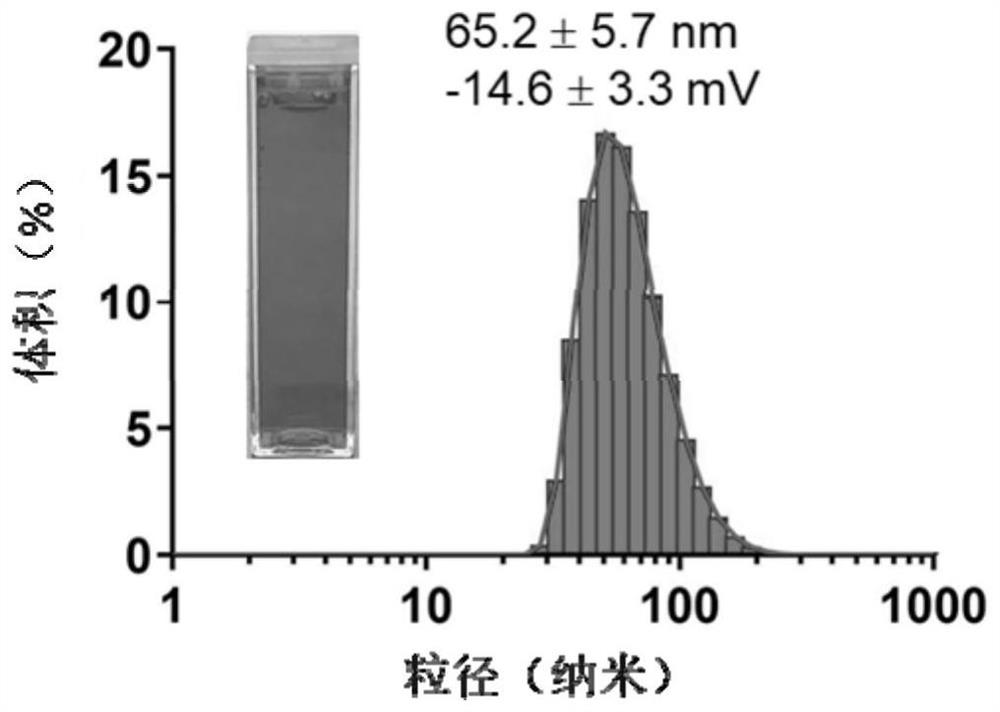
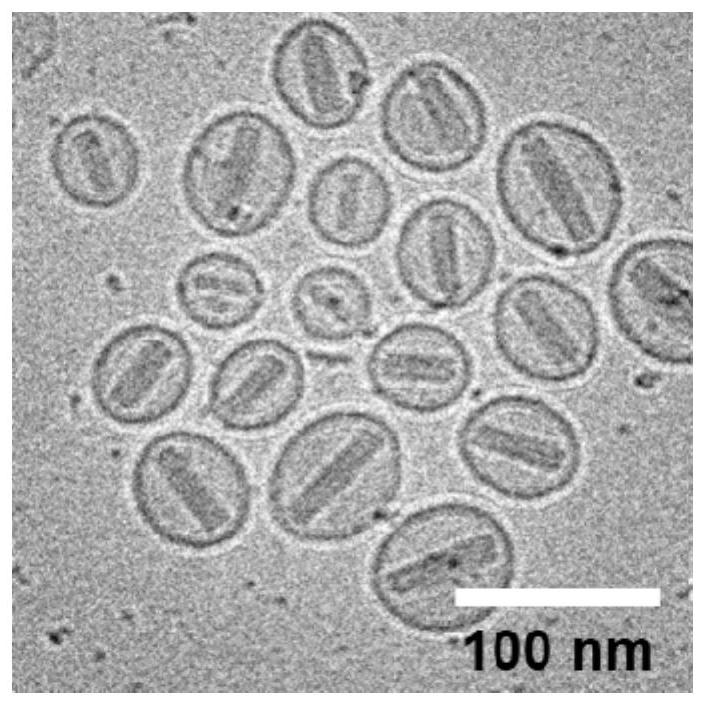
[0088] Example 2: Preparation and Characterization of GCSDL Liposomes and Control CCDL Liposomes
[0089] Adopt ammonium sulfate gradient method to prepare GCSDL liposome, the preparation process is as follows:
[0090] 1) In a 250mL flask, mix HSPC (hydrogenated soybean lecithin) (23.6mg, 0.03mmol), CHOL (cholesterol) (7.7mg, 0.02mmol) and DOPE-GSH (glutathione) in a molar ratio of 3:2:3 Peptide-modified dioleoylphosphatidylethanolamine) (43.0mg, 0.03mmol) was dissolved in chloroform (10mL) and rotovaped under reduced pressure to form a lipid film.
[0091] 2) Add ammonium sulfate solution (0.3M, 20mL), and after hydration under ultrasonic, extrude the solution on a polycarbonate filter membrane (100nm) to obtain a blank liposome solution. ) at 5000r / min, centrifuge for 60min to remove excess ammonium sulfate solution.
[0092] 3) Mix the concentrated liposomes with DOX (doxorubicin) (mass ratio: 16:2) in 4.6 mL of histidine buffer (10 mM, pH 6.5) containing 10% (w / w) sucro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com