Synthesis method of 2-hydroxy-5-nonylpropiophenone and application of 2-hydroxy-5-nonylpropiophenone in copper extraction agent
A synthesis method and technology of copper extractant, which are applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of carbonyl compounds by hydrolysis, etc., can solve the problems of low product yield and purity, unstable synthesis process, and low conversion rate of finished products, etc. problems, to achieve high conversion rate and product purity, low stability in use, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
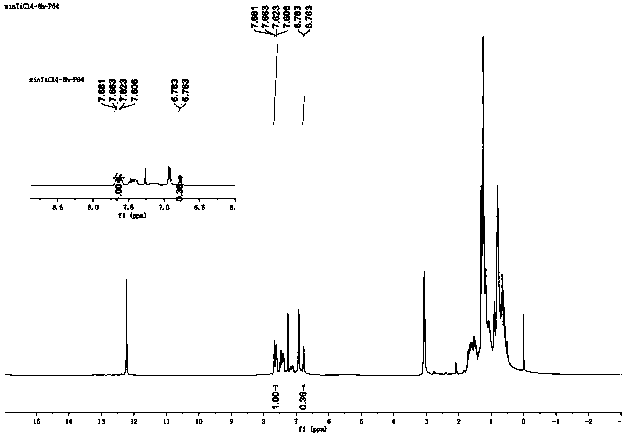
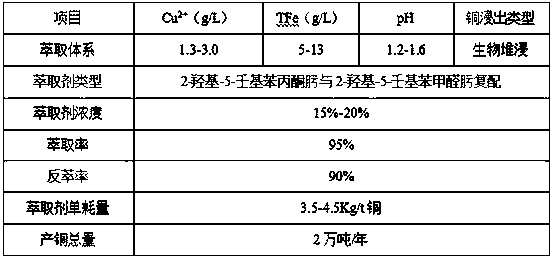
Image
Examples
Embodiment 1
[0021] The present invention provides a kind of technical scheme: the synthetic method of 2-hydroxyl-5-nonylpropiophenone and the application in copper extractant, the synthetic method of 2-hydroxyl-5-nonylpropiophenone comprises the following steps:
[0022] S1, esterification reaction, in a 500ml three-neck flask equipped with magnetic stirring, reflux condenser and HCl absorption device, 4-nonylphenol: propionyl chloride: tetrachloroethylene according to the ratio of 1:1.1:1.5 (molar ratio), Add propionyl chloride dropwise to nonylphenol tetrachloroethylene at room temperature under stirring, and react at 50°C for 5 hours to generate nonylphenol ester;
[0023] S2, rearrangement reaction, nonylphenol ester: TiCl4 is heated to reflux (120°C) according to 1:1 (molar ratio) and kept for 10 hours for ortho rearrangement reaction;
[0024] S3, hydrolysis reaction, hydrolyze with 6N HCl and 2N HCl at 50°C-70°C for 1 hour in sequence, then wash with water until pH 6.0 and separate...
Embodiment 2
[0028] The present invention provides a kind of technical scheme: the synthetic method of 2-hydroxyl-5-nonylpropiophenone and the application in copper extractant, the synthetic method of 2-hydroxyl-5-nonylpropiophenone comprises the following steps:
[0029] S1, esterification reaction, in a 500ml three-neck flask equipped with magnetic stirring, reflux condenser and HCl absorption device, 4-nonylphenol: propionyl chloride: tetrachloroethylene according to the ratio of 1:1.1:1.5 (molar ratio), Add propionyl chloride dropwise to nonylphenol tetrachlorethylene at room temperature under stirring, and react at 53°C for 5 hours to generate nonylphenol ester;
[0030] S2, rearrangement reaction, nonylphenol ester: TiCl4 is heated to reflux (120°C) according to 1:1 (molar ratio) and kept for 11 hours for ortho rearrangement reaction;
[0031] S3, hydrolysis reaction, hydrolyze with 6N HCl and 2N HCl at 50°C-70°C for 1.3h in sequence, then wash with water until pH 6.0 and separate ph...
Embodiment 3
[0035] The present invention provides a kind of technical scheme: the synthetic method of 2-hydroxyl-5-nonylpropiophenone and the application in copper extractant, the synthetic method of 2-hydroxyl-5-nonylpropiophenone comprises the following steps:
[0036] S1, esterification reaction, in a 500ml three-neck flask equipped with magnetic stirring, reflux condenser and HCl absorption device, 4-nonylphenol: propionyl chloride: tetrachloroethylene according to the ratio of 1:1.1:1.5 (molar ratio), Add propionyl chloride dropwise to nonylphenol tetrachlorethylene at room temperature under stirring, and react at 55°C for 5 hours to generate nonylphenol ester;
[0037] S2, rearrangement reaction, nonylphenol ester: TiCl4 is heated to reflux (120°C) according to 1:1 (molar ratio) and kept for 12 hours for ortho rearrangement reaction;
[0038] S3, hydrolysis reaction, hydrolyze with 6N HCl and 2N HCl at 58°C for 2.3 hours in sequence, then wash with water until pH 6.0 and separate phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com