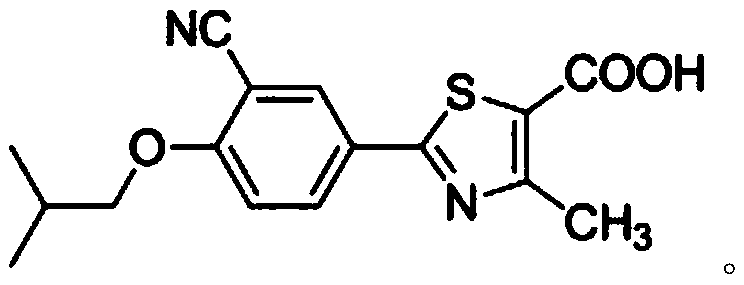
Synthesis process of febuxostat
A technology of febuxostat and synthesis process, which is applied in the field of febuxostat synthesis process, can solve the problems of high operational risk, high production cost, and unsuitability for industrial production, and achieve safe operation, high purity of intermediate products, and improved The effect of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
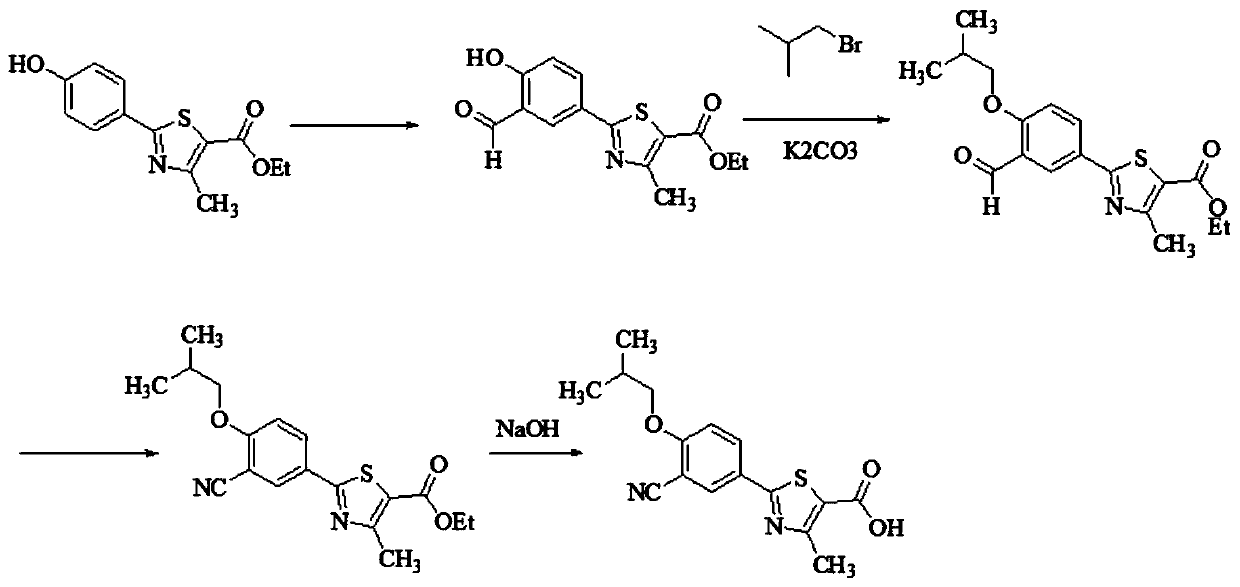
[0026] The synthetic technique of febuxostat of the present invention comprises the following steps:
[0027] S1. Preparation of 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5 from ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate - ethyl formate;
[0028] S2, 2-(3-formyl-4-isobutoxyphenyl)-4- Ethyl methylthiazole-5-carboxylate;
[0029] S3, prepare 2-(3-cyano-4-isobutoxyphenyl) with ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate - ethyl 4-methylthiazole-5-carboxylate;
[0030] S4, preparing febuxostat crude product with ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate;
[0031] S5. The pure product of febuxostat is obtained by monocrystalline the crude product of febuxostat, and the pure product of febuxostat is the target product.
[0032] Synthetic route such as figure 1 As shown, the present invention uses raw materials different from the original synthetic route, without using highly toxic cuprous cyanide and potassium ...
Embodiment 2
[0034] Based on Example 1, the specific steps of S1 are as follows: according to the amount of feed in Table 1, add polyphosphoric acid and phosphoric acid in turn in the reaction tank, start stirring and heating, and add 2-(4-hydroxyphenyl)- Ethyl 4-methylthiazole-5-carboxylate and urotropine, control the heating rate, slowly raise the temperature to 60-90°C, stir and dissolve completely, keep warm for about 2-6 hours, take samples for testing, after the reaction is completed, cool down Add 10L of drinking water after the temperature is below 30°C, stir for 1 hour, cool down to 0-5°C, centrifuge, wash the filter cake twice with drinking water, put the filter cake in a blast drying oven, and dry it at 50-80°C. Obtain 340-360 g of ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate with a purity of ≥98%.
[0035] Table 1 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-formic acid ethyl ester preparation dosage
[0036] Material name Feed amount (g) ...
Embodiment 3
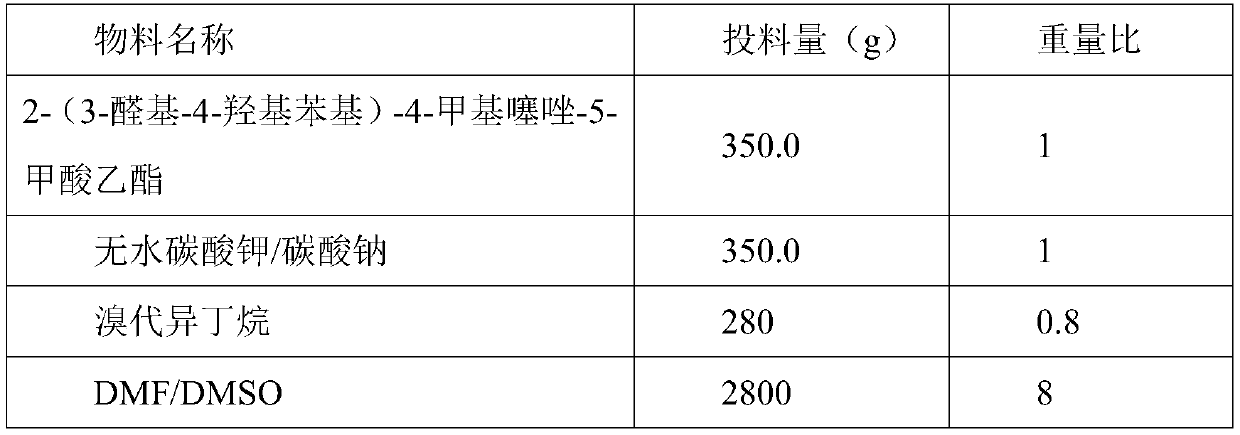
[0038] Based on the above examples, the specific steps of S2 are as follows: according to the amount of feed in Table 2, add DMF or DMSO in the reaction tank, start stirring, add 2-(3-formyl-4-hydroxyphenyl)-4-methanol Ethylthiazole-5-carboxylate, stir to dissolve, add anhydrous potassium carbonate or sodium carbonate, stir and add bromoisobutane dropwise, dropwise for 1~3h, keep the temperature at 30~60°C, after the dropwise addition, Slowly raise the temperature to 70-100°C, keep it warm for 2-3 hours, after the reaction is completed, start adding hydrochloric acid dropwise, check the pH value, stop adding hydrochloric acid when the pH is between 7-8, turn on the cold brine and cool down to -5-5°C, Stir and crystallize for 2h, centrifuge, wash, and dry the filter cake at 60-70°C to obtain ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate 380- 400g, the purity is greater than 98.0%.
[0039] Table 2 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-formic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com