Ethyl sulfate artificial antigen and preparation method and application thereof
An ethyl sulfate, artificial antigen technology, applied in the preparation of sulfate, the preparation method of peptide, chemical instruments and methods, etc. problem, to achieve the effect of high accuracy, high specificity and specificity, and accurate test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
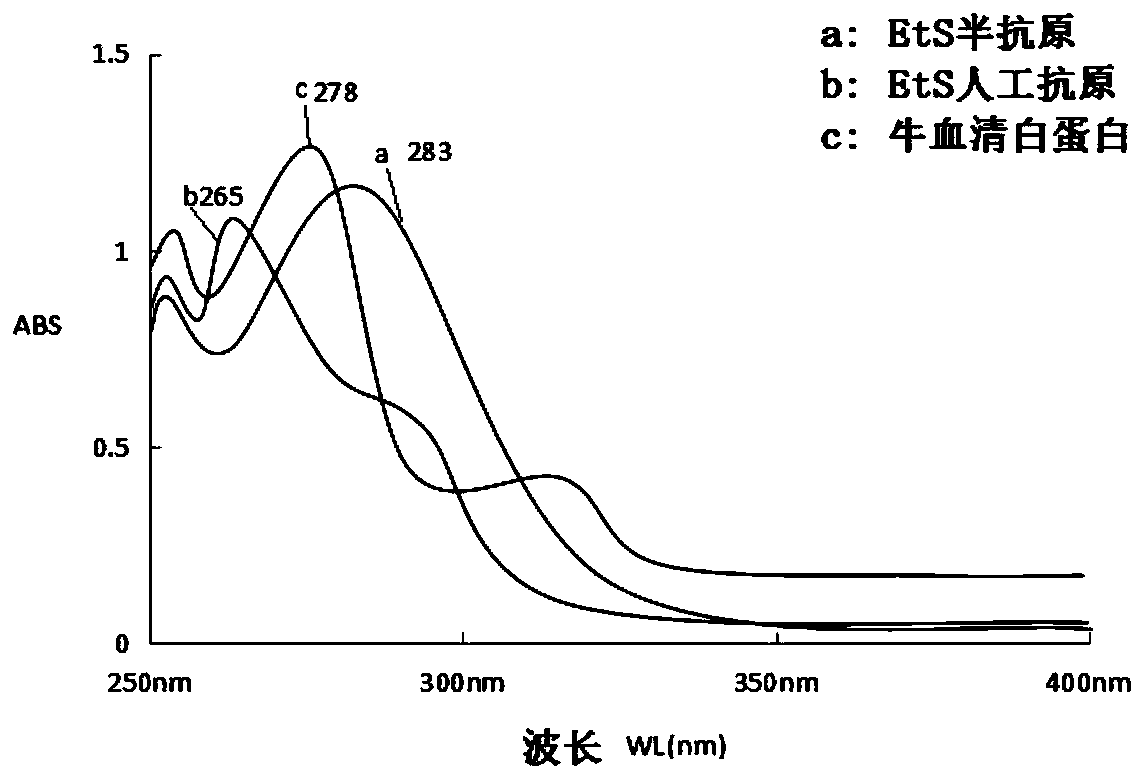
Image
Examples
Embodiment 1
[0036] (1) Preparation of ethylsulfate hapten
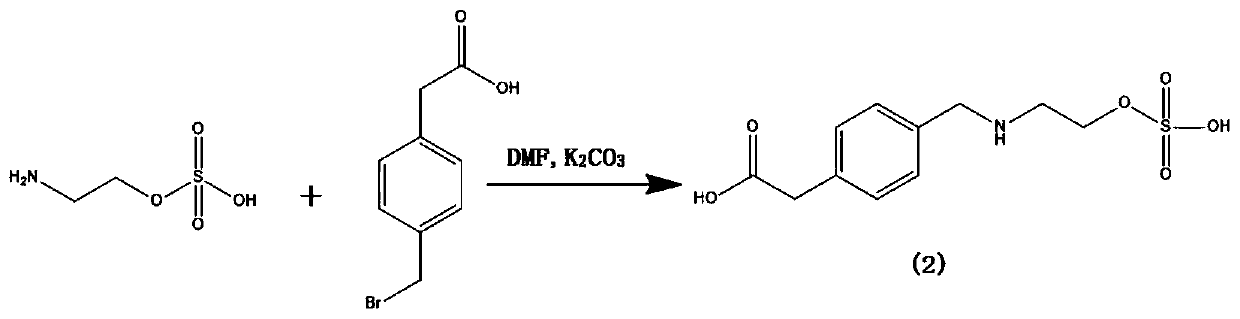
[0037] Weigh 100mg (0.71mmol) 2-aminoethanol hydrogen sulfate, join in 50ml one-necked round bottom flask, then add 5mlN, N-dimethylformamide (DMF), 276mg (2mmol) potassium carbonate and 300mg (1.31 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, add a stirring bar, and stir the reaction at room temperature for 20 hours; Dichloromethane:methanol=1:1 (volume ratio), product ratio shift value Rf=0.3~0.4; obtain hapten (2) 86mg, specific synthetic route is as follows figure 1 shown.
[0038] (2) Preparation of ethyl sulfate artificial antigen
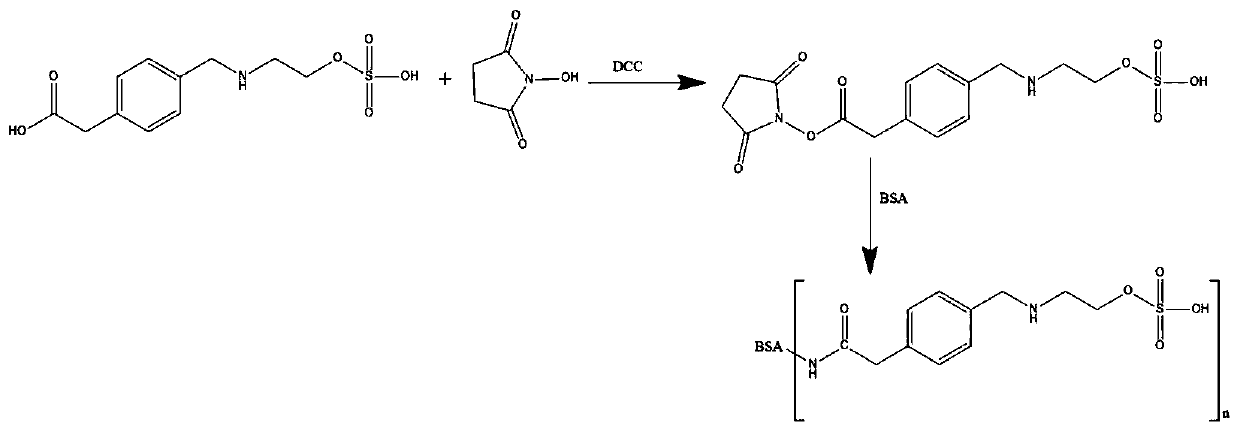
[0039] a. Weigh 50mg (0.17mmol) ethyl sulfate hapten in a 50ml round bottom flask, add 2.5ml N,N-dimethylformamide (DMF), then add 39mg (0.34mmol) N-hydroxysuccinyl Imine (NHS) and 70 mg (0.34 mmol) N,N-dicyclohexylcarbodiimide (DCC) were stirred at room temperature overnight, centrifuged after the reaction, and the supernatant was taken as solution A.
[0040] b. Weigh 14.5 g of disodium hyd...
Embodiment 2
[0053] (1) Preparation of ethylsulfate hapten
[0054] Weigh 100mg (0.71mmol) of 2-aminoethanol hydrogen sulfate and add it to a 50ml single-necked round bottom flask, then add 5mlN,N-dimethylformamide (DMF), 138mg (1mmol) potassium carbonate and 200mg (0.87 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, add a stirring bar, and stir the reaction at room temperature for 20 hours; Dichloromethane:methanol=1:1 (volume ratio), product ratio shift value Rf=0.3~0.4; obtain 40mg of hapten (2), the specific synthetic route is as follows figure 1 shown.
[0055] (2) Preparation of ethyl sulfate artificial antigen
[0056] a. Weigh 40mg (0.14mmol) ethyl sulfate hapten in a 50ml round bottom flask, add 2ml N,N-dimethylformamide (DMF), then add 24mg (0.21mmol) N-hydroxysuccinyl Amine (NHS) and 43mg (0.21mmol) N,N-dicyclohexylcarbodiimide (DCC), stirred at room temperature overnight, centrifuged after the reaction, and the supernatant was taken as solution A.
[0057] b. Weigh 14.5 g of d...
Embodiment 3
[0063] (1) Preparation of ethylsulfate hapten
[0064] Weigh 100mg (0.71mmol) 2-aminoethanol hydrogen sulfate, join in 100ml single-necked round bottom flask, then add 10mlN,N-dimethylformamide (DMF), 552mg (4mmol) potassium carbonate and 600mg (2.62 mmol) 2-(4-(bromomethyl)phenyl)acetic acid, add a stirring bar, and stir the reaction at room temperature for 20 hours; Dichloromethane:methanol=1:1 (volume ratio), product ratio shift value Rf=0.3~0.4; obtain hapten (2), the specific synthetic route is as follows figure 1 shown.
[0065] (2) Preparation of ethyl sulfate artificial antigen
[0066] a. Weigh 50mg (0.17mmol) of ethyl sulfate hapten in a 100ml round bottom flask, add 10ml of N,N-dimethylformamide (DMF), then add 1.1mmol of N-hydroxysuccinimide (NHS) and 1.0 mmol N,N-dicyclohexylcarbodiimide (DCC), stirred at room temperature overnight, centrifuged after the reaction, and the supernatant was taken as liquid A.
[0067] b. Weigh 14.5 g of disodium hydrogen phosphat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com