Chondroitin sulfate ABC lyase mutant and secretory expression method thereof
A chondroitin sulfate and mutant technology, applied in the field of bioengineering, can solve the problems of low intracellular expression and inability to meet the production requirements of chondroitin sulfate oligosaccharide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
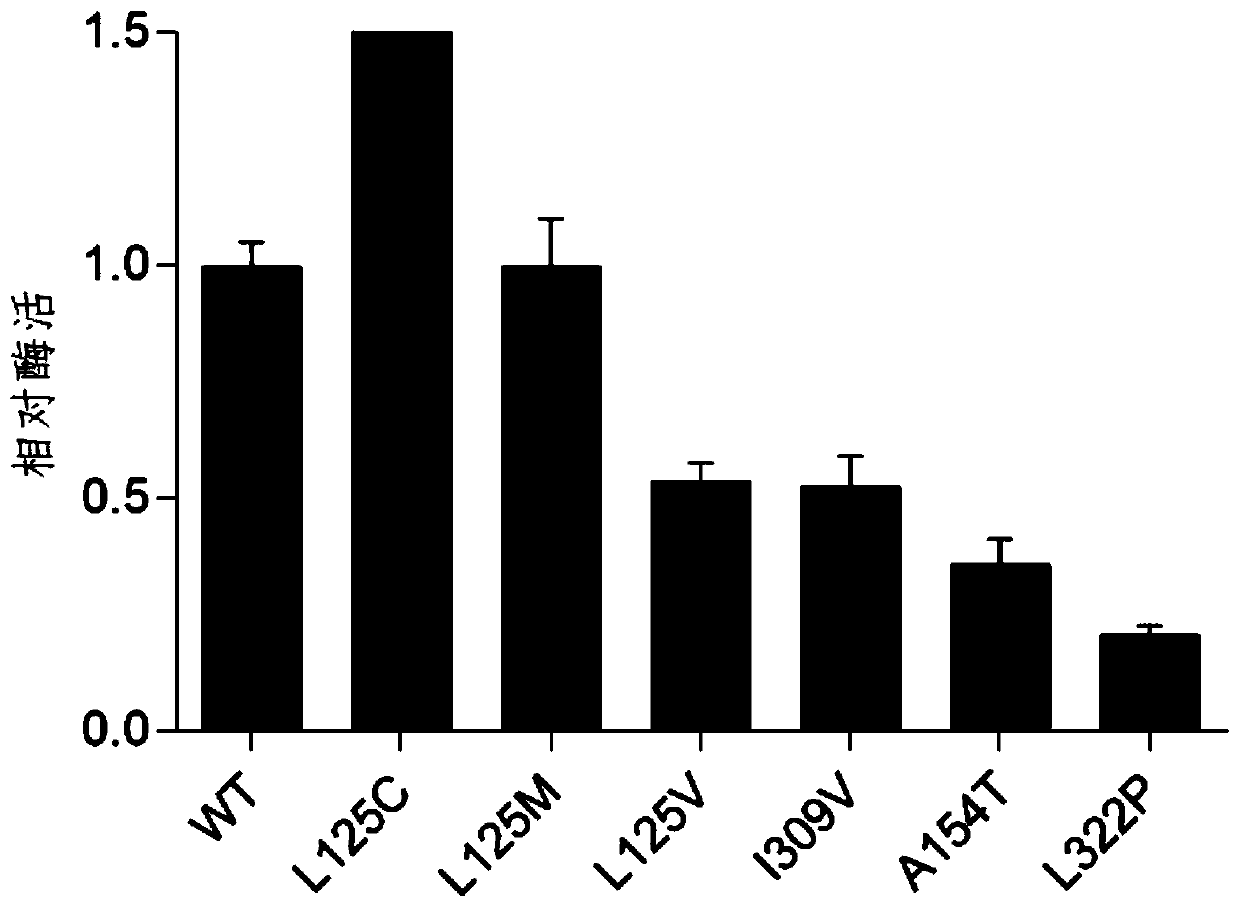
[0029] The preparation method of embodiment 1 chondroitin sulfate ABC lyase mutant
[0030] The chondroitinase ABC derived from Proteus vulgaris was synthesized, and the original DNA sequence of the encoded chondroitinase ABC is shown in SEQ ID NO.1. The 125th, 154th, 322nd, and 309th amino acid positions were selected by sequence comparison, and saturation mutations were carried out respectively. A standard PCR program was used and the primers used are listed in Table 1. Link the gene encoding the original chondroitinase ABC or the gene obtained after PCR mutation to the pET-28a plasmid XhoI and NdeI restriction sites, and introduce it into Escherichia coli BL21 (DE3) to obtain the expression of the original chondroitinase Recombinant E. coli strains of ABC or chondroitinase ABC mutants. The obtained recombinant strain was inoculated in LB medium, cultured overnight at 37°C, transferred to a 250mL Erlenmeyer flask filled with LB medium, cultured at 30°C for 1 hour, induced ...
Embodiment 2
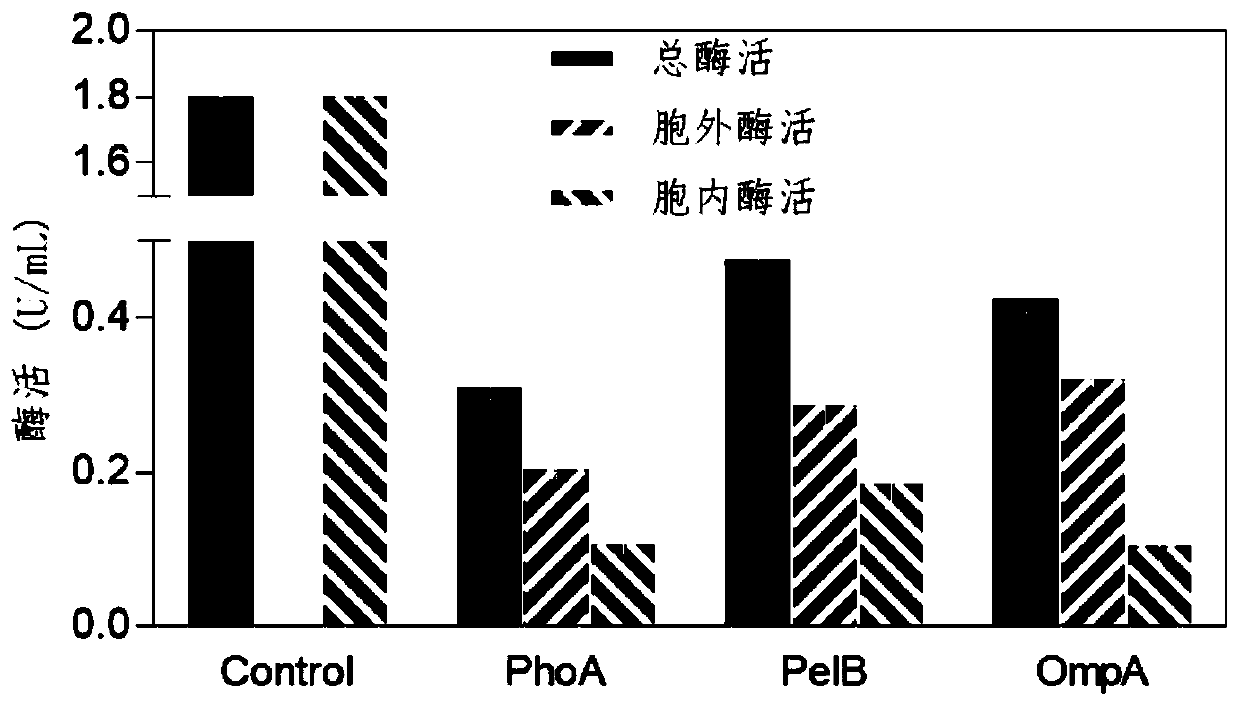
[0035] Example 2 Fusion of enzyme gene and signal peptide to realize extracellular secretion expression
[0036] Experimental process: The signal peptides PelB, PhoA, and OmpA were cloned from the Escherichia coli BL21 genome, and fused into the genes encoding the mutants, so that the N-terminus of the translated amino acid sequence was fused with the signal peptide. The resulting fusion gene was connected to the XhoI and NdeI restriction sites of the pET-28a plasmid, and introduced into Escherichia coli BL21 (DE3) to obtain a recombinant Escherichia coli strain. The recombinant bacteria expressing the gene without fusion signal peptide were used as the control group. The obtained recombinant strain was inoculated in LB medium, cultured overnight at 37°C, transferred to a 250mL Erlenmeyer flask filled with LB medium, cultured at 30°C for 1 hour, induced by adding IPTG with a final concentration of 0.5mM, and placed at 27°C Cultivate for 20h.
[0037] The collection method of...
Embodiment 3
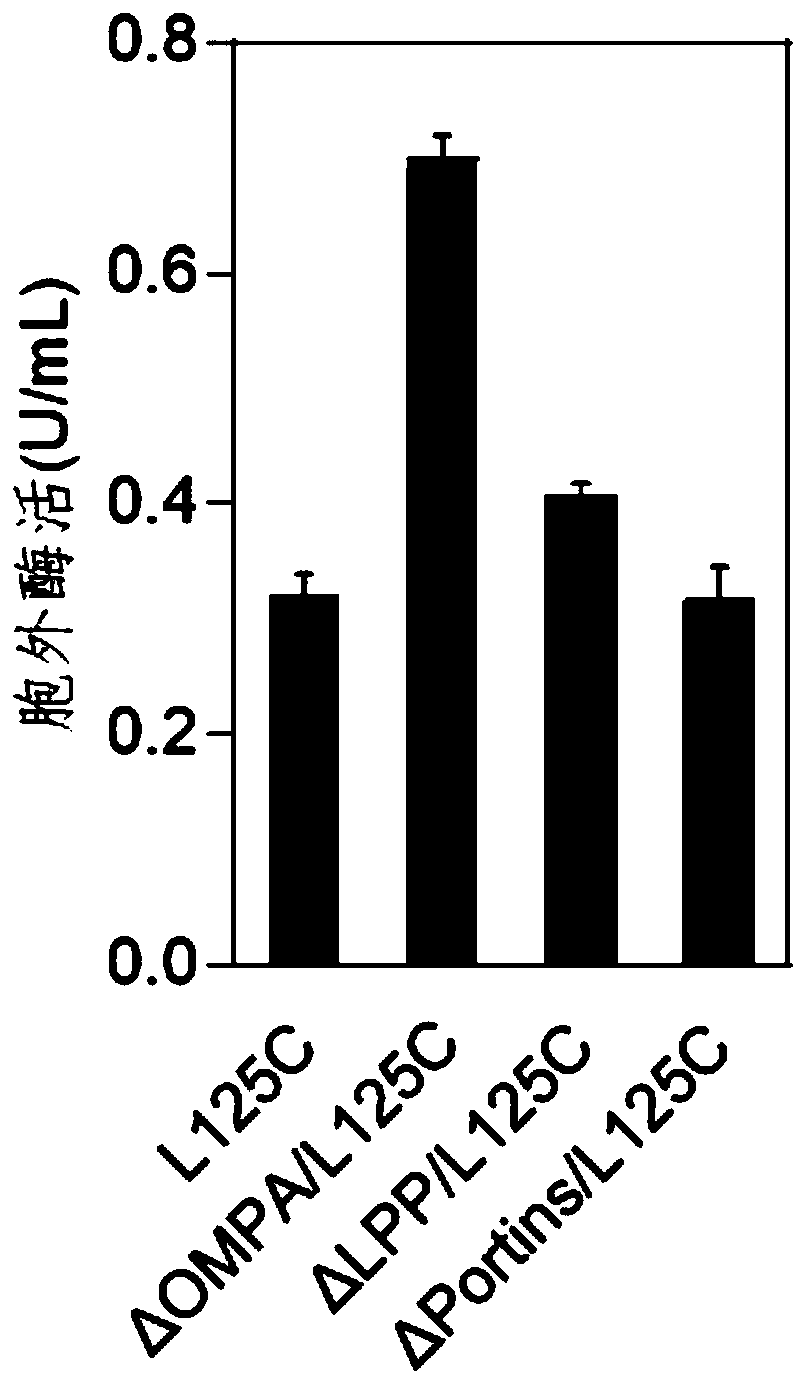
[0040] Example 3 Knocking Out the Outer Membrane Protein of the Bacterial Strain Improves the Extracellular Secretion Expression of the Enzyme
[0041] Experimental process: transfer pCas9 plasmid into BL21(DE3) competent cells, apply kanamycin plate, and culture at 30°C. A single colony was extracted from the above plate, inoculated in SOB medium containing kanamycin, and cultured overnight at 30°C for 12 hours. Transfer to SOB liquid medium containing kanamycin with 2% inoculum, and culture to OD at 30°C 600 When it is 0.1, add arabinose at a final concentration of 10mmol / L to induce the expression of the recombinase. Continue to cultivate until the OD is 0.6, ice-bath the bacterial solution for 10 minutes, centrifuge at 4°C and 4000r / min for 5 minutes, and collect the bacterial cells. The bacterial cells were washed 3 times with pre-cooled 10% glycerol, and concentrated 100 times to prepare competent cells for electroporation.
[0042] The pET-28a plasmid connected with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com