Photocatalytic foliage fertilization method
A technology of foliar fertilization and photocatalysis, which is applied in the agricultural field to achieve the effect of improving nitrogen utilization rate, simple fertilization method, and strong ammonia production persistence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
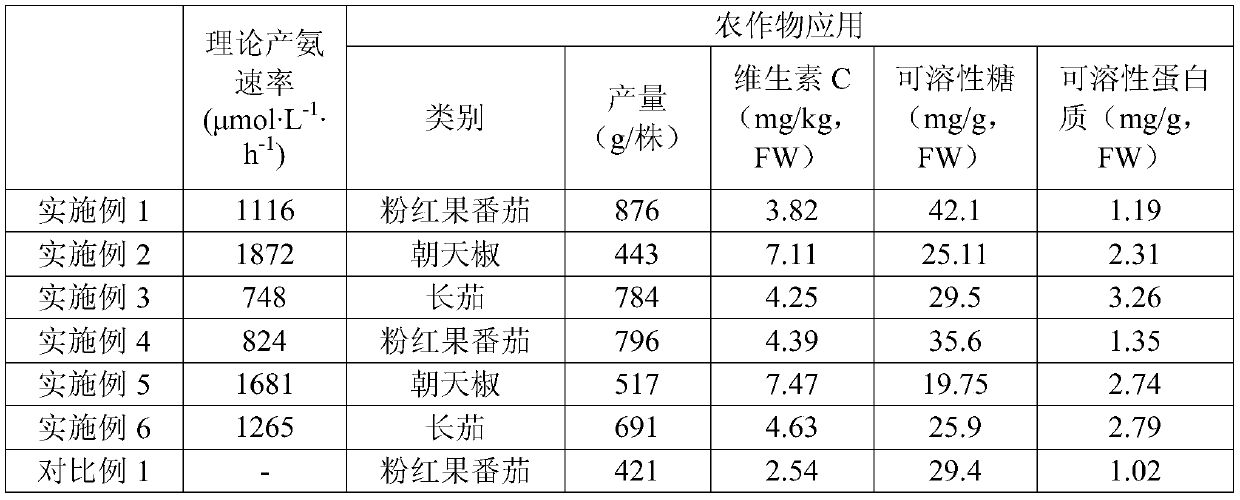
Examples
Embodiment 1
[0042] Photocatalyst preparation:
[0043] Take 3 parts of HF solution (≥40wt%) and add it dropwise to 25 parts of tetrabutyl titanate, stir and mix for 1 hour, seal the solution and move it into an oven, and keep it at 180°C for 24 hours. After cooling, filter and dry at 60°C to obtain TiO 2 .
[0044] Take 1 part TiO 2 The catalyst is ultrasonically dispersed in 100 parts of water, 0.1 part of urea is added, stirred and mixed for 1 hour, the solution is sealed and moved into an oven, and kept at 120°C for 12 hours. After cooling, filter and dry at 60°C to obtain photocatalyst N / TiO 2 .
[0045] Preparation of ammonia solution and glycerol solution:
[0046] Quantitative glycerol was weighed and dissolved in water to prepare a glycerol solution, wherein the volume ratio of glycerol was 5%. Weigh quantitative N / TiO 2 The catalyst is dissolved in glycerol solution to prepare an ammonia supply solution, wherein the concentration of the catalyst is 250mg / L.
[0047] Deter...
Embodiment 2
[0052] Photocatalyst preparation:
[0053] Take 3 parts of HF solution (≥40wt%) and add it dropwise to 25 parts of tetrabutyl titanate, stir and mix for 1 hour, seal the solution and move it into an oven, and keep it at 180°C for 24 hours. After cooling, filter and dry at 60°C to obtain TiO 2 .
[0054] Take 1 part TiO 2 Catalyst, ultrasonically dispersed in 100 parts of water, adding 0.4 parts of FeCl 2 , Stir and mix for 1h, seal the solution and move it into an oven, and keep it at 100°C for 12h. After cooling, filter and dry at 60°C to obtain photocatalyst Fe / TiO 2 .
[0055] Preparation of ammonia solution and butanetriol solution:
[0056] Quantitative butanetriol was weighed and dissolved in water to prepare a butanetriol solution, wherein the volume ratio of butanetriol was 5%. Weigh quantitative Fe / TiO 2 The catalyst is dissolved in butanetriol solution and prepared as an ammonia supply solution, wherein the concentration of the catalyst is 500 mg / L.
[0057]...
Embodiment 3
[0062] Catalyst preparation:
[0063] Take 7 parts of zinc acetate nonahydrate and 11 parts of sodium hydroxide, dissolve in 100 parts of water, stir and mix for 1 hour, seal the solution and move it into an oven, and keep it at 100°C for 12 hours. After cooling, filter and dry at 60°C to obtain ZnO.
[0064] Take 0.2 parts of GO and ultrasonically disperse it in 100 parts of water, add 1 part of ZnO catalyst, stir and mix for 1 h, seal the solution and move it into an oven, and keep it at 180 °C for 12 h. After cooling, filter and dry at 60°C to obtain rGO / ZnO.
[0065] Preparation of ammonia solution and ethylene glycol solution:
[0066] Weigh a certain amount of ethylene glycol and dissolve it in water to prepare an ethylene glycol solution, wherein the volume ratio of ethylene glycol is 6%. Quantitative rGO / ZnO catalyst was weighed and dissolved in ethylene glycol solution to prepare ammonia supply solution, wherein the catalyst concentration was 700mg / L.
[0067] Det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com