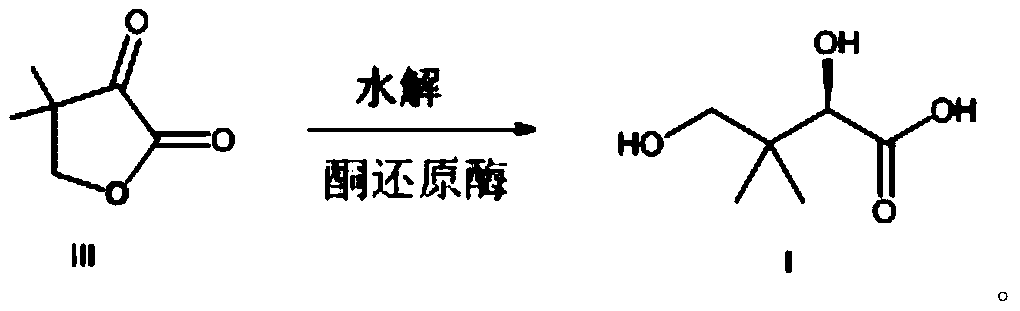
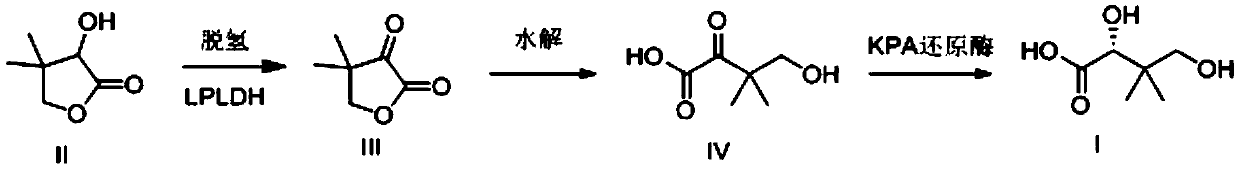
Preparation method for D-pantoic acid
A technology of pantoic acid and dimethylbutyric acid, which is applied in the field of preparation of D-pantoic acid, can solve the problems of complex synthetic route operation, inappropriate preparation conditions, and reduced production costs, so as to achieve high process stability and avoid The use of polluting heavy metals and toxic reagents, and the effect of fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
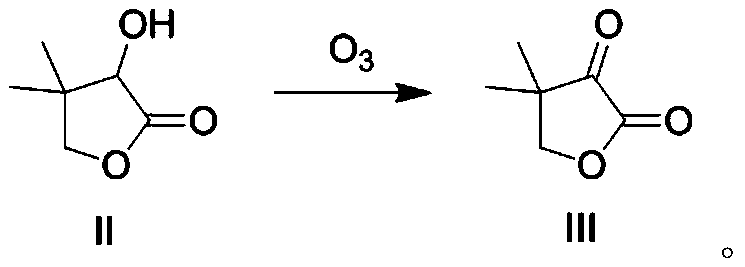
[0038] Add 80mL tetrahydrofuran and 13.01g (0.1mol) of raw material 3-hydroxy-4,4-dimethyldihydrofuran-2-one to a 250mL reaction flask, then add 4.0g NaOH (0.1mol), and control the temperature for -10 below ℃, followed by O3 15 minutes (20g / h), control the temperature at -20°C to 20°C for 12h, and GC (gas chromatography) detects that the reaction of the raw materials is complete. Add concentrated HCl (2M) to the system, adjust the pH to about 7, and continue to add 50mL of water solution. The aqueous phase was extracted twice with 50mLEA (ethyl acetate) (50ml was used for each extraction), the organic phases were combined, dried by adding 10.0g of anhydrous sodium sulfate, filtered with suction, and concentrated under reduced pressure to obtain compound III (12.16g). 95%, HPLC assay purity 98.12%. Compound III carries out proton nuclear magnetic spectrum and carbon nuclear magnetic spectrum analysis, and the data are as follows:
[0039] 1 H NMR (400H Z ,DMSO-d6): δ4.37(s...
Embodiment 2
[0045] Add 80mL tetrahydrofuran and 13.01g (0.1mol) of raw material 3-hydroxy-4,4-dimethyldihydrofuran-2-one to a 250mL reaction flask, then add 20.0g NaOH (0.5mol), and control the temperature for -10 below ℃, followed by O 3 15 minutes (20g / h), control the temperature at -20°C to 20°C for 12h, and GC detects that the reaction of the raw materials is complete. Add concentrated HCl (2M) to the system, adjust the pH to about 7, and continue 50mL of water solution. The aqueous phase was extracted twice with EA (50ml*2, that is, 50ml for each extraction), the organic phases were combined, dried by adding about 10.0g of anhydrous sodium sulfate, filtered with suction, and concentrated under reduced pressure to obtain compound III (12.33g). The yield was 97%, and the purity was 98.5% as determined by HPLC. Compound III carries out proton nuclear magnetic spectrum and carbon nuclear magnetic spectrum analysis, and the data are as follows:
[0046] 1 H NMR (400H Z ,DMSO-d6): δ4...
Embodiment 3
[0052] Add 80mL tetrahydrofuran and 6.5g (0.05mol) of raw material 3-hydroxy-4,4-dimethyldihydrofuran-2-one to a 250mL reaction flask, then add 10.0g NaOH (0.25mol), and control the temperature for -10 below ℃, followed by O 3 36 minutes (20g / h), control the temperature at -20°C to 20°C for 12h, and GC detects that the reaction of the raw materials is complete. Add concentrated HCl (2M) to the system, adjust the pH to about 7, and continue to add 50mL of water solution. The aqueous phase was extracted twice with EA (50ml*2, that is, 50ml for each extraction), the organic phases were combined, dried by adding about 6.0g of anhydrous sodium sulfate, filtered with suction, and concentrated under reduced pressure to obtain compound III (6.15g). The yield was 97%, and the purity was 99.2% as determined by HPLC. Compound III carries out proton nuclear magnetic spectrum and carbon nuclear magnetic spectrum analysis, and the data are as follows:
[0053] 1 H NMR (400H Z ,DMSO-d6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com