A kind of conjugate of immunoactive peptide-biliverdin, its preparation method and application
An immunoactive peptide and biliverdin technology, applied in the field of immunoactive peptide-biliverdin conjugates, can solve the problems of unresearched biological safety performance, immune-related adverse effects, short half-life of small molecules, etc., so as to reduce tumor metastasis. The effect of relieving and eliminating tumor inflammation and remodeling tumor inflammatory microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
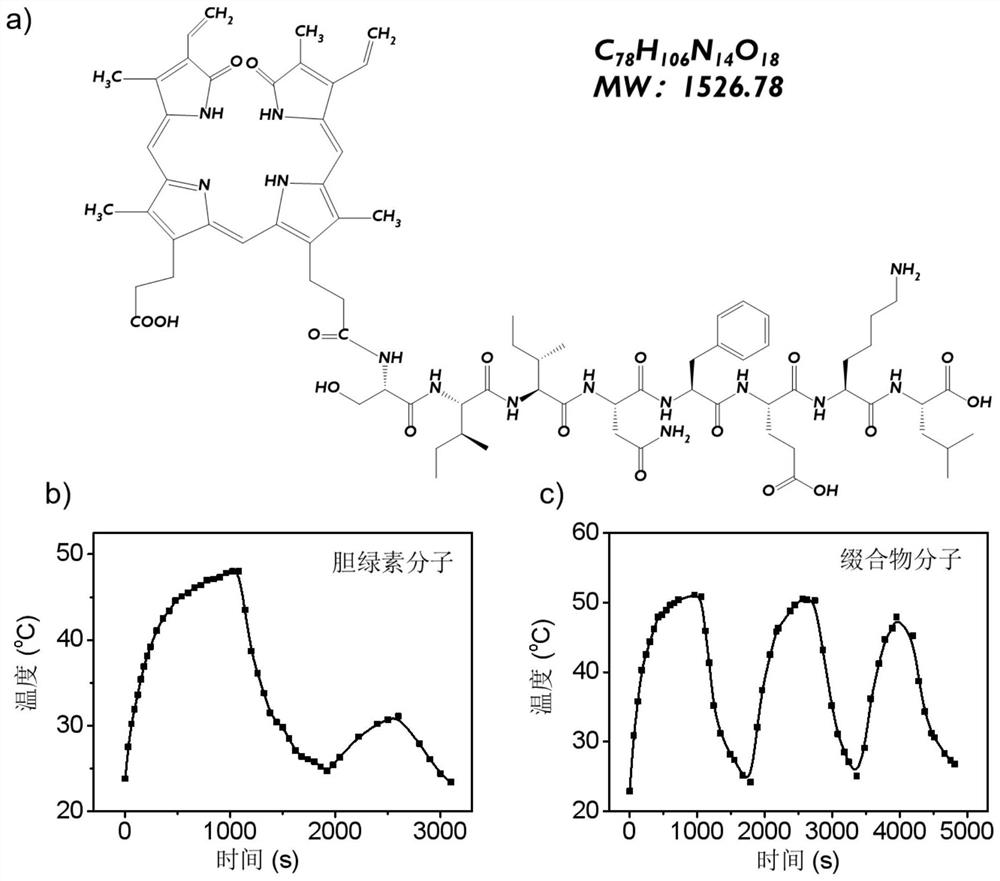
[0135] According to the following steps, the biliverdin-SIINFEKL conjugate was obtained by chemical synthesis: Weigh a certain amount of biliverdin, EDC·HCl, NHS, and DMF, add them to the reactor in turn and mix them uniformly; put the obtained mixture at room temperature Stir in the dark for 24 hours; then add water to stir and collect the precipitate; add anhydrous DMF to the obtained precipitate and mix evenly, then add SIINFEKL peptide and anhydrous triethylamine, and stir at room temperature for 24 hours in the dark; collect the above reaction The precipitate was purified by size exclusion chromatography; the resulting material was recrystallized to obtain the pure molecular conjugate. Among them, the concentration of biliverdin was 100 mM, the concentration of EDC·HCl was 100 mM, the concentration of NHS was 50 mM, and the concentration of peptide was 200 mM. The 1HNMR information of the prepared conjugate is as follows:
[0136]1HNMR (600MHz) δ=11.95~13.00(3H),10.68(s,...
Embodiment 2
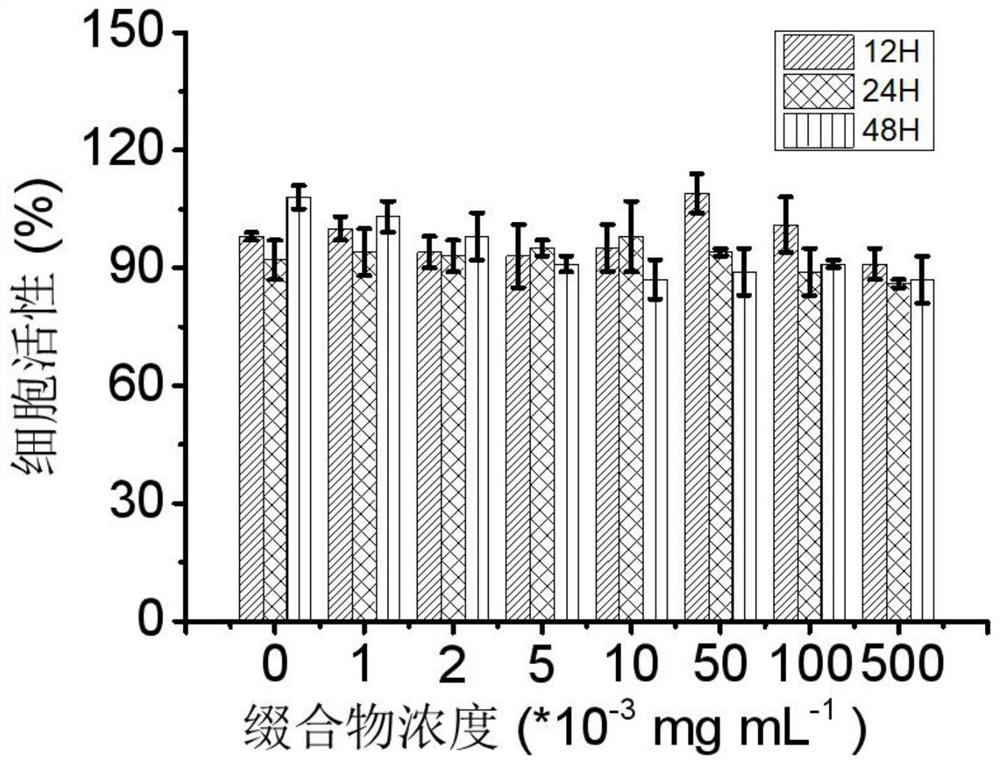
[0139] The biliverdin molecule and SIINFEKL were synthesized according to the chemical synthesis method in Example 1 to prepare a biliverdin-SIINFEKL conjugate. A certain mass of conjugate was weighed, pre-dissolved in a small amount of DMSO solution, and then directly dissolved in PBS solution, stirred and dissolved, then filtered and sterilized, and the pH value was adjusted to neutral. The prepared conjugates with different concentration gradients were co-incubated with human umbilical vein endothelial cells HUVEC in the dark, and the biological safety of the conjugates was evaluated by MTT colorimetry. figure 2 The results of the cell activity experiment of the conjugate prepared in Example 2 show that the prepared conjugate has high biological safety and has no obvious cytotoxicity to human umbilical vein endothelial cells HUVEC.
Embodiment 3
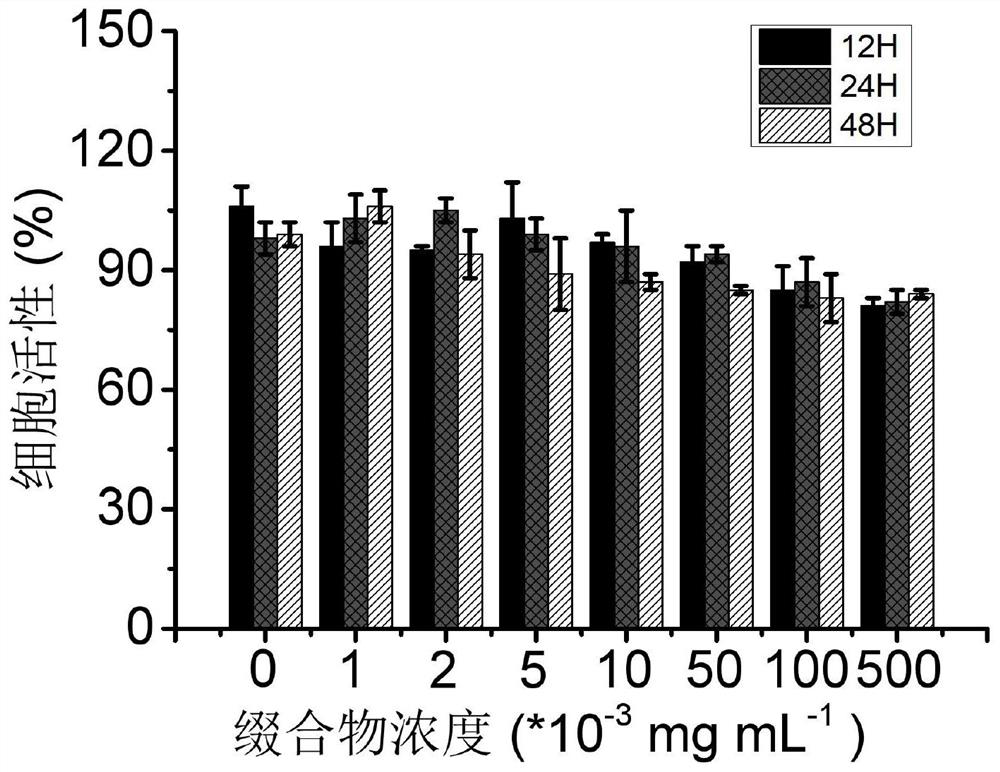
[0141] First, biliverdin and excess zinc acetate were chemically synthesized to obtain biliverdin-Zn metal complexes. The experimental method was as follows: biliverdin and excess zinc acetate were dissolved in methanol solution, stirred at 60°C for 4 hours, and the obtained The solution was rotary evaporated to remove the solvent to obtain a solid, and the obtained solid was purified by a reverse-phase chromatographic column to obtain the biliverdin-Zn complex, wherein the mass concentration ratio of biliverdin to zinc acetate was 1:5. The biliverdin-Zn metal complex and NYSKPTDRQYHF were prepared according to the aforementioned chemical synthesis method to obtain the biliverdin-Zn-NYSKPTDRQYHF conjugate. A certain mass of conjugate was weighed, pre-dissolved in a small amount of DMSO solution, and then directly dissolved in PBS solution, stirred and dissolved, then filtered and sterilized, and the pH value was adjusted to neutral. The prepared conjugates with different conce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| laser intensity | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com