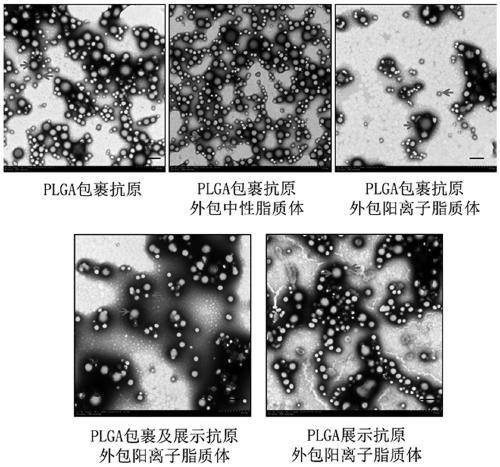
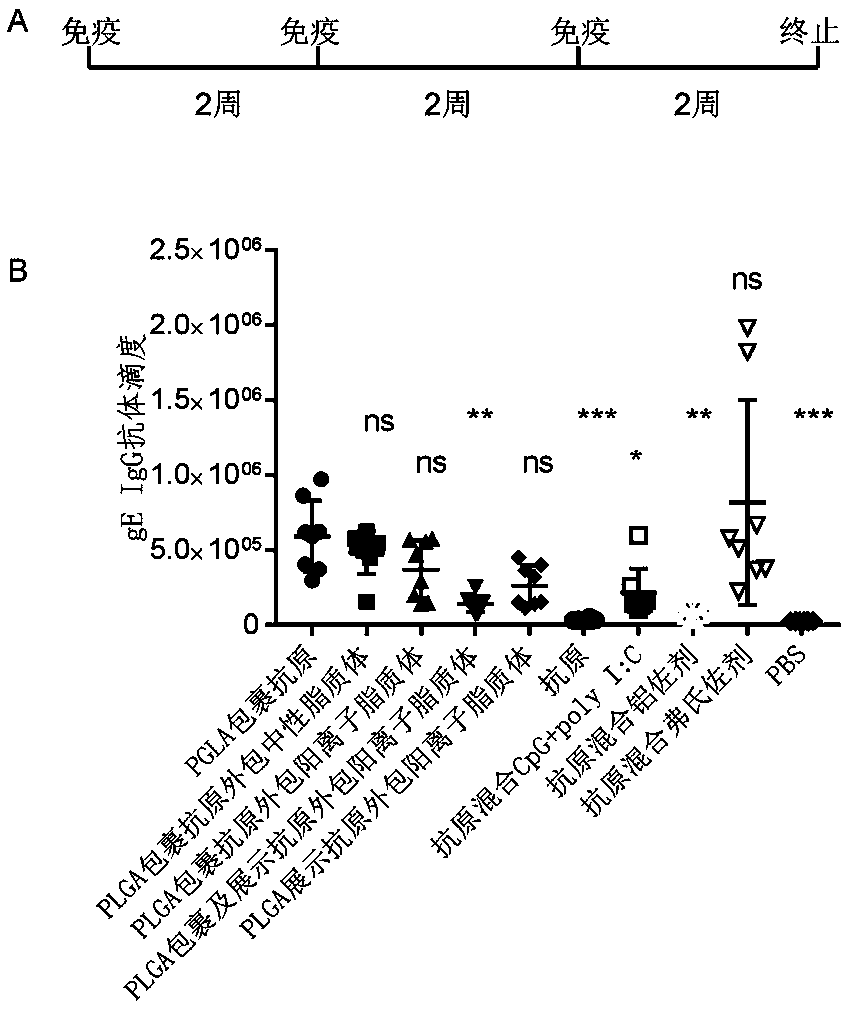
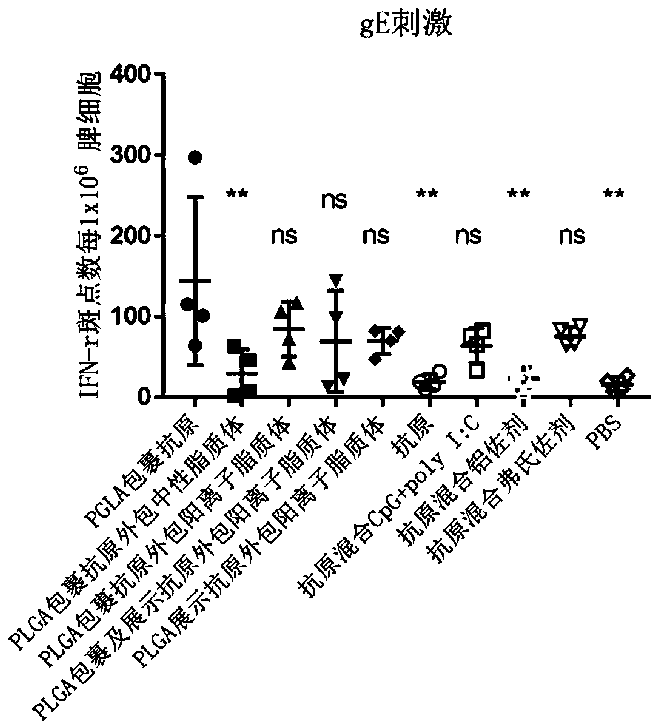
Herpes zoster vaccine composition as well as preparation method and application thereof
A vaccine composition and herpes zoster technology, applied in the field of vaccines, can solve the problems of temperature sensitivity of active ingredients, expensive vaccines, and limited sources, so as to avoid the side effects of systemic inflammation and promote the effect of cellular immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1—Weighed 0.5 mg of gE extracellular region glycoprotein expressed by CHO (purchased from Pujian Biotechnology Co., Ltd.), dissolved in 2.5 ml of PBS and mixed evenly to obtain the mixed preparation group ①Ag.
Embodiment 2
[0036] Example 2—weigh 0.5 mg of gE, 0.25 mg of non-thio-oxidized forms CpG BW006 and CpG 2395 (synthesized from Shanghai Sangon Bioengineering Co., Ltd.), 1.25 mg of LMW PolyI:C (purchased from InvivoGen), and dissolve in Mix 2.5 ml of PBS evenly to obtain the mixed preparation group ②Ag+CpG+Poly I:C.
Embodiment 3
[0037]Example 3 - 0.5 mg of gE was weighed, dissolved in 1.25 ml of PBS, and mixed with an equal volume of aluminum adjuvant (purchased from ThermoFisher) to obtain the mixed preparation group ③Ag+Alum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com