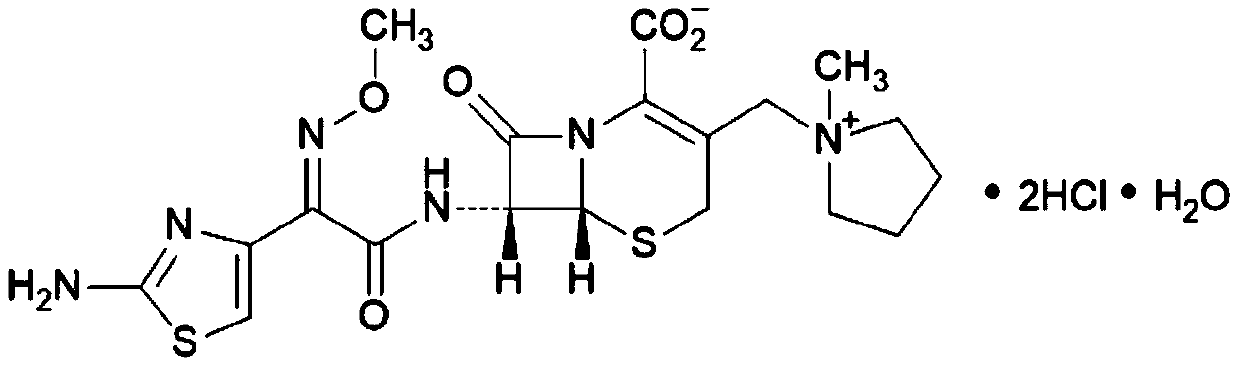
Cefepime hydrochloride medicinal preparation for treating new indications of tympanitis
A technology of cefepime hydrochloride and preparations, which is applied in the field of drug preparation, can solve problems such as allergic reactions, pharmacological hazards, and unfavorable human health, and achieve the effect of improving the safety of use and reducing impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1 Synthesis of Cefepime Hydrochloride
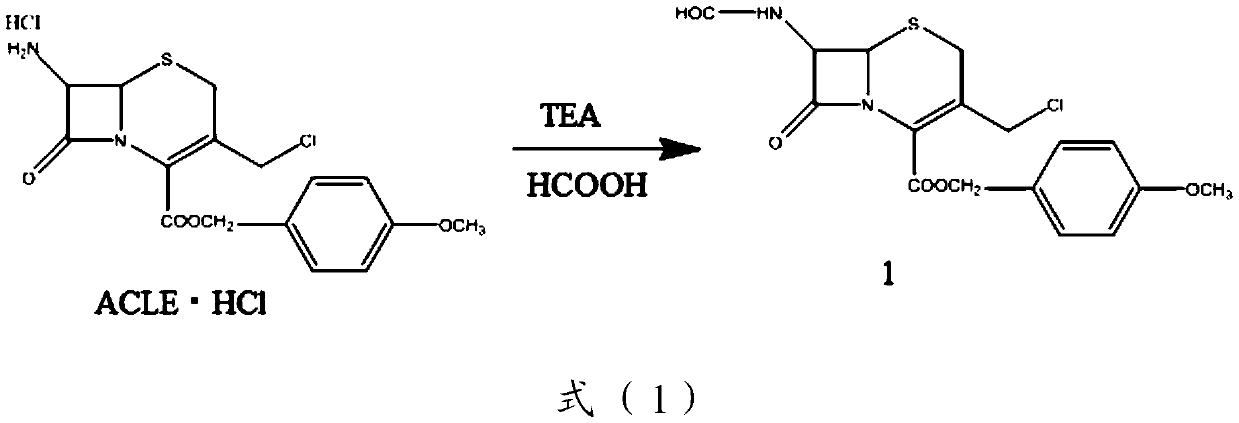
[0097] Preparation of compound 1:
[0098] Add 160g of ACLE·HCl and 320mL of tetrahydrofuran into the reaction vessel in turn, add 55ml (0.39mol) of triethylamine dropwise under ice bath, after the drop is complete, remove the ice bath, and stir at room temperature for 1 h. Filter, wash with 150mL tetrahydrofuran for 3 times, and the filtrate is set aside
[0099] In another reaction vessel, add 44ml of formic acid and 110ml (1.17mol) of acetic anhydride, and stir at 40-50°C for 0.5h. Cool down, stir in an ice bath for 1 h, filter, and wash with ethanol to obtain 119 g of compound 1 with a yield of 86%.
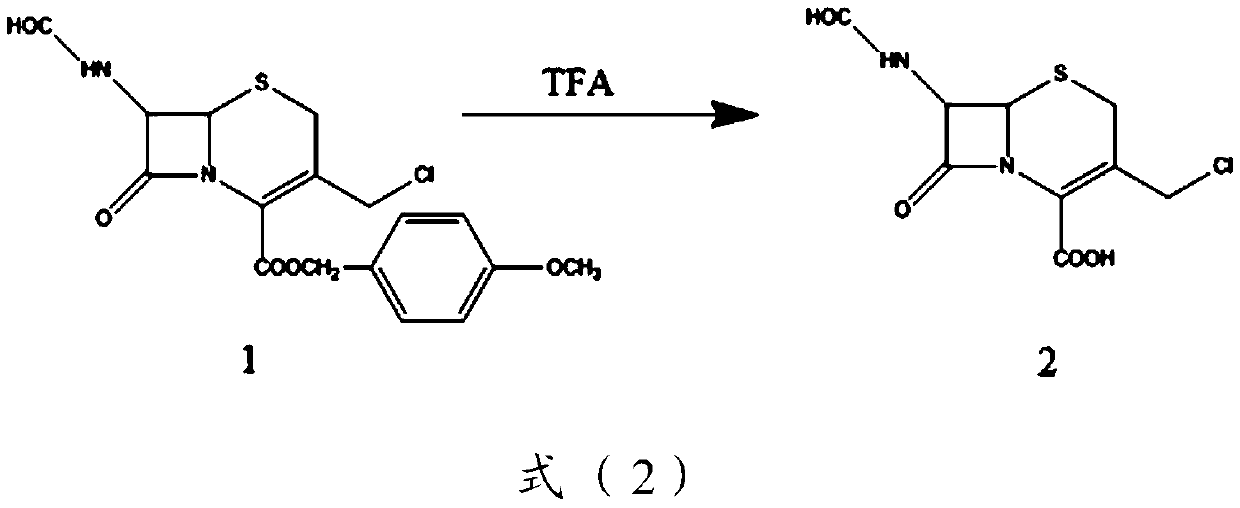
[0100] Preparation of Compound 2:
[0101] Add 110g of compound 1, 330mL of dichloromethane-210ml of anisole (volume ratio 3:2) into the reaction vessel in turn, add 213ml of trifluoroacetic acid dropwise in ice bath, stir at room temperature for 1 hour, pour the reaction solution into the container In a reaction fla...
Embodiment 2
[0108] Example 2 Synthesis of Cefepime Hydrochloride
[0109] The synthesis process is the same as in Example 1, the only difference is that in the synthesis of compound 1, ethyl acetate is used as the reaction solvent I. Finally, 74.7 g of compound 1 was prepared, with a yield of 54%, indicating that tetrahydrofuran as the reaction solvent I is beneficial to the improvement of the conversion rate of raw materials compared with ethyl acetate.
Embodiment 3~4
[0110] Embodiment 3~4 Synthesis of Cefepime Hydrochloride
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com