Use of 2-(ethylaminomethyl)-5-(phenyl)furan in the preparation of medicines for inhibiting tlr7/8
A technology of ethylaminomethyl and phenyl, applied in the field of TLRs receptor inhibitors, can solve problems such as side effects of TLR agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
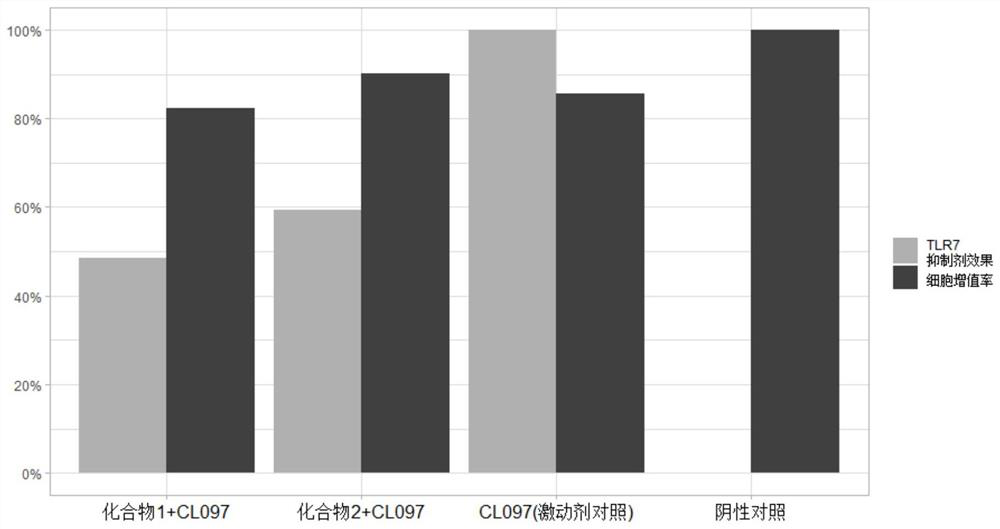
[0039] Effect of compound 1, compound 2 and control compound TLR7 / 8 activator on the inhibitory activity of TLR7 / 8 protein in HEK293 cells and the cell proliferation rate measured by CCK-8
[0040] Solution: HEK-Blue TM -hTLR7 cells (HEK293 cells transfected with humanized TLR7, purchased from InvivoGen) were seeded in 96-well plates at a density of 4×10 4 To each well, agonist CL097 (5 μM) and test compound 1 or compound 2 (40 μM) were added overnight to obtain supernatant. The obtained supernatant was aspirated with a pipette gun, the supernatant (20 μL) was tested by Quanti-blue (OD 630 nm), and the residual liquid was tested for cell proliferation rate by CCK-8. If the cell proliferation rate is high, it means that it is less toxic to cells and the cells can grow normally.
[0041] Index of Quanti-Blue value=(A-B) / (C-B); where A: the average value of the wells to be tested; B: the average value of the matrix containing cells; C: the positive control (CL097).
[0042] St...
Embodiment 2
[0054] NO activity test and cell survival experiment of compound 1 / compound 2
[0055] Protocol: RAW264.7 cells were seeded in 96-well plates at a density of 4×10 4 One per well, compound 1, compound 2 or indomethacin (40 μM) and lipopolysaccharide (LPS) (1 μg / mL, 10 μL / well) were added after 1.5 h. After 24h, the NO concentration was tested by Griess reagent (540nm); the cell growth was tested by MTT (200μL, 0.5mg / mL, 450nm).
[0056] Steps:
[0057] ① Culture RAW264.7 cells in DMEM medium (10% FBS + 1% penicillin / streptomycin), 37°C, 5% CO 2 ; After the cells grew stably, they were seeded in 96-well plates (5×10 4 per well), overnight.
[0058] ②Add compound (40 μM, 100 μL) and then add LPS (1 μg / mL, 10 μL / well).
[0059] ③The next day, take 50 μL of the cell supernatant and use the Griess kit (nitric oxide kit, purchased from Beyond Biotechnology Co., Ltd.) to draw a standard curve at the same time.
[0060] ④ Discard the supernatant, add MTT [3-(4,5-dimethylthiazole-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com