Anti-arthritis medicine composition
An anti-arthritis and composition technology, applied in the field of medicine, can solve the problems of little effect, achieve the effects of reducing toxic side effects, high heart safety, and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
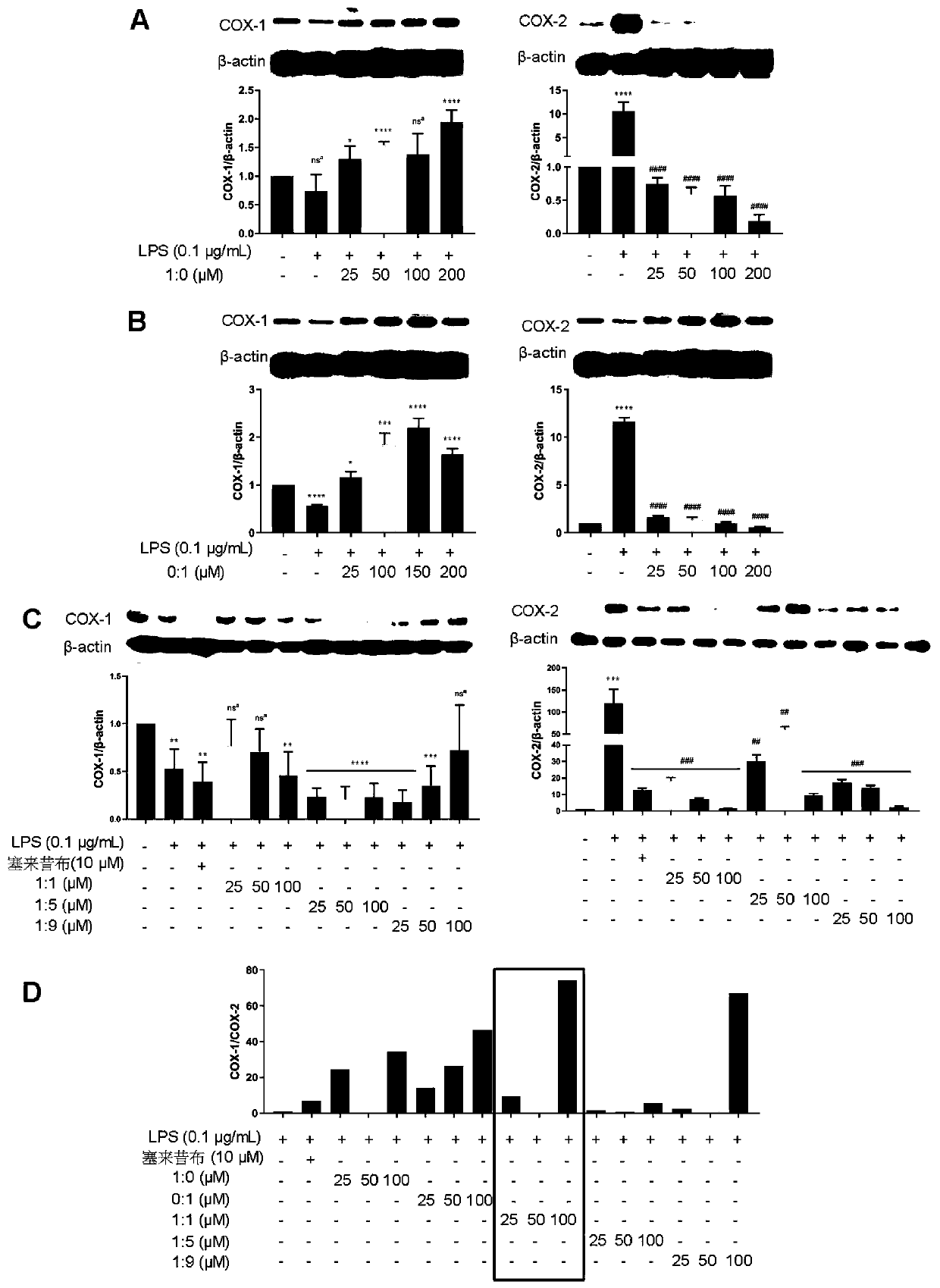
[0040] Embodiment 1: the preparation of pharmaceutical composition (for research in vitro)
[0041] Take scopoletin 1.9216g, add dimethyl sulfoxide (DMSO) 5mL, mix and set aside, as the mother solution of scopoletin (2M). Take 3.7237g of syringin, add 5mL of DMSO, and mix well for later use as the mother solution of syringin (2M). Preparation of scopoletin and syringin in different ratios of substances for in vitro studies:
[0042] (1) 1:0
[0043] Dilute scopoletin mother solution with DMEM medium to 12.5, 25, 50, 100, 150, 200 μM;
[0044] (2) 0:1
[0045] Dilute syringin mother solution with DMEM medium to 12.5, 25, 50, 100, 150, 200 μM;
[0046] (3) 1:1
[0047] Scopoletin and syringin mother liquor were mixed at a ratio of 1:1 to obtain a mixture, and the solute concentration of the mixture was diluted with medium to 12.5, 25, 50, 100, 150, 200 μM;
[0048] (4)1:5
[0049] Scopoletin and syringin mother liquor were mixed at a ratio of 1:5 to obtain a mixture, and ...
Embodiment 2
[0052] Example 2: Preparation of pharmaceutical composition (for in vivo studies)
[0053] Different mass ratios of scopoletin and syringin were prepared for in vivo studies:
[0054] (1) 1:0
[0055] Take scopoletin 10g, add 5% CMC-Na 50mL, dilute to 200mg / mL solution;
[0056] (2) 0:1
[0057] Take 10g of syringin, add 5% CMC-Na 50mL, and dilute to 200mg / mL solution;
[0058] (3) 1:1
[0059] Take 5 g each of scopoletin and syringin, add 50 mL of 5% CMC-Na, and dilute to a 200 mg / mL solution.
Embodiment 3
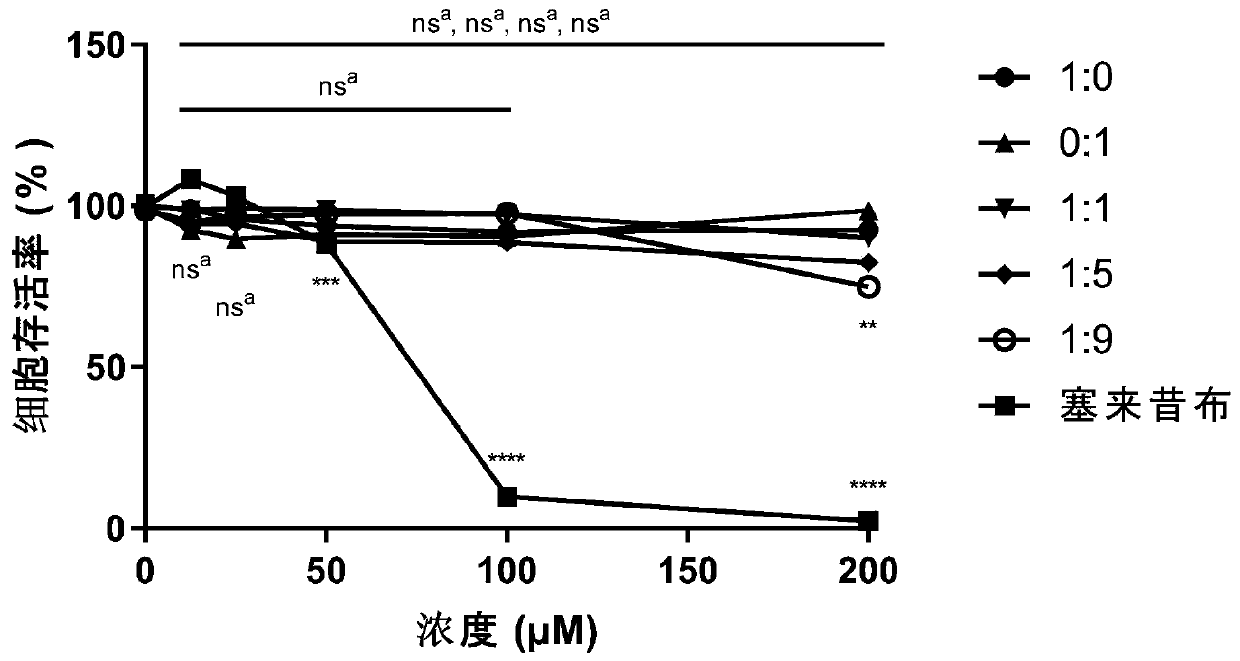
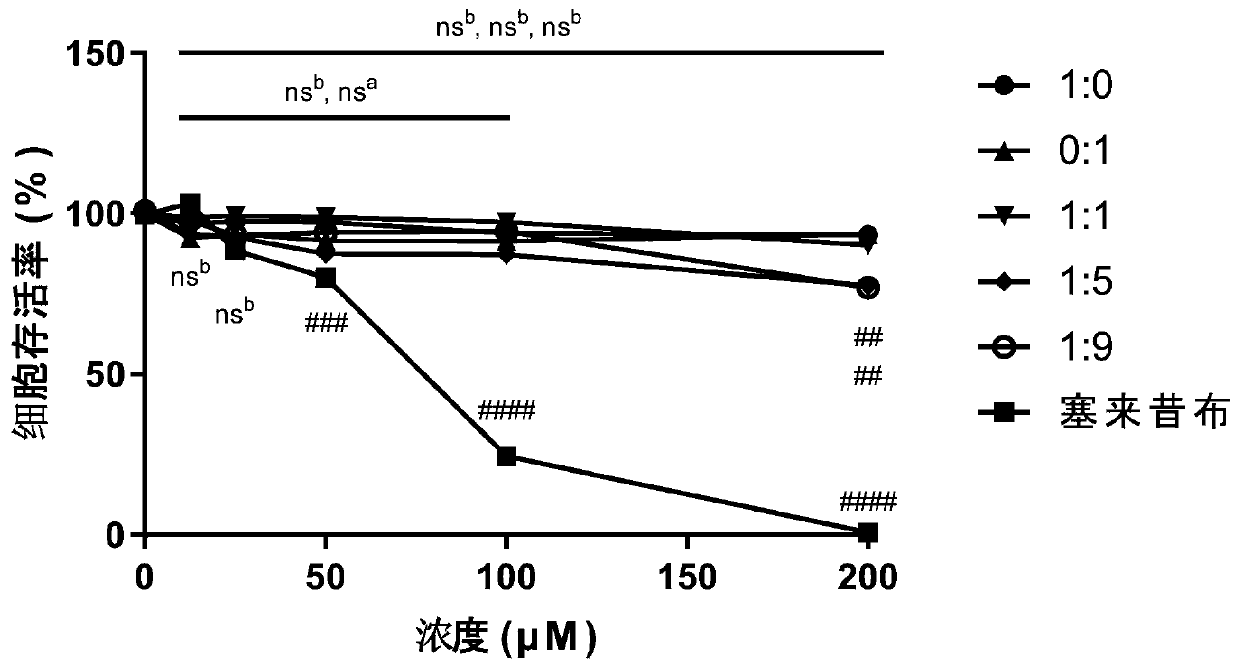
[0060] Embodiment 3: the in vitro myocardial safety test of pharmaceutical composition
[0061]Cardiomyocytes H9c2 were seeded in 96-well plates (5000 cells / well). After 24 hours, the cells were congested, and different ratios of scopoletin and syringin (1:0, 0:1, 1:1, 1:1) were added. 5, 1:9; the concentration of each ratio is 12.5, 25, 50, 100, 200 μM) / celecoxib (12.5, 25, 50, 100, 200 μM) and incubated for 24 hours. Using the culture medium as a negative control and celecoxib as a positive control, the cell survival rate is detected by the MTT method, and then the cytotoxic effect of the pharmaceutical composition is evaluated.
[0062] Dissolve 30 mg of MTT powder in 6 mL of phosphate-buffered saline (PBS) in the dark, add 10 μL to each well, and incubate in the incubator for 2 h in the dark. Then centrifuge (1000rpm, 10min), carefully suck off the supernatant, add 100μL DMSO to each well, and place on a shaker for 10min at low speed to fully dissolve the crystals. The a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com