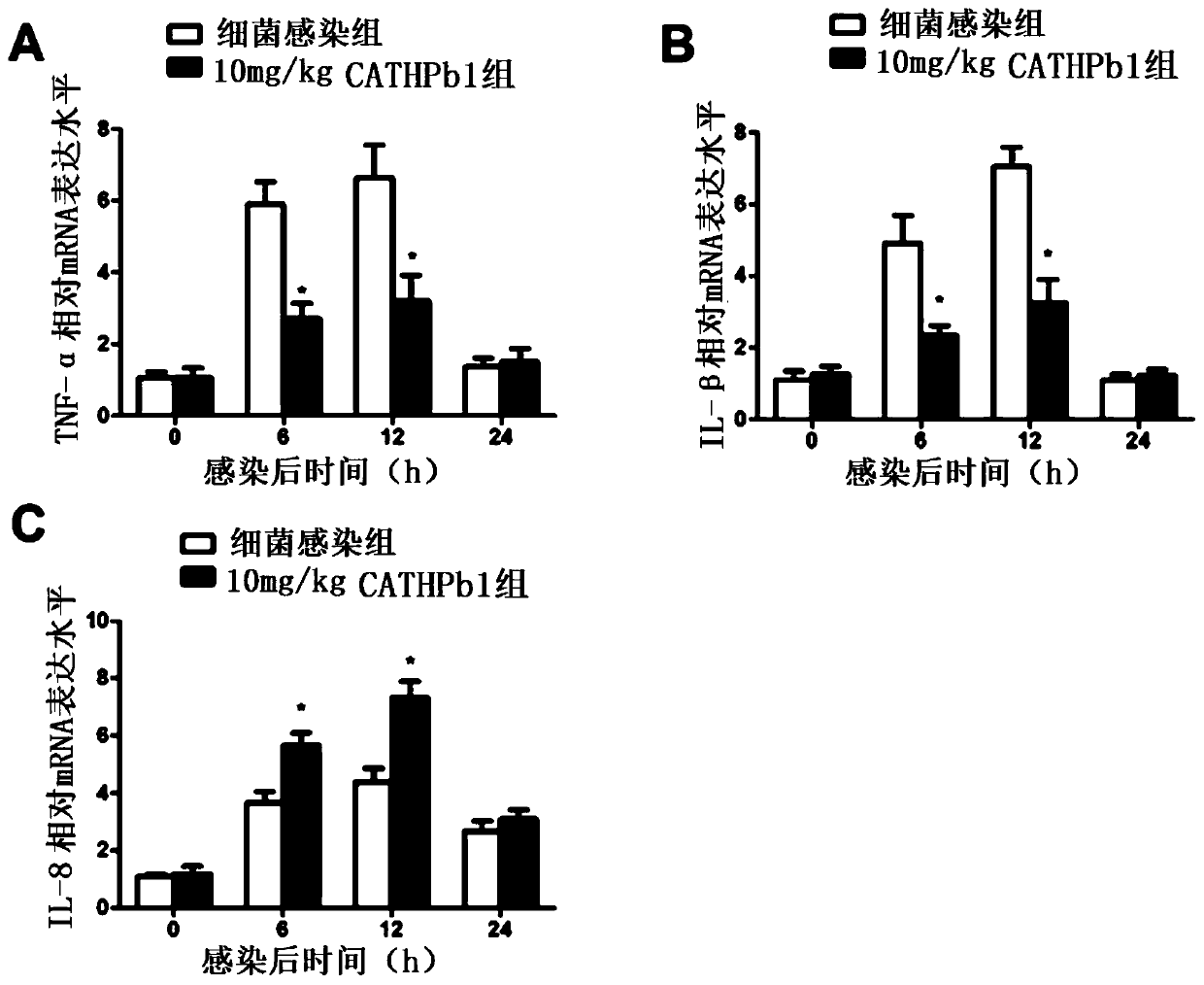
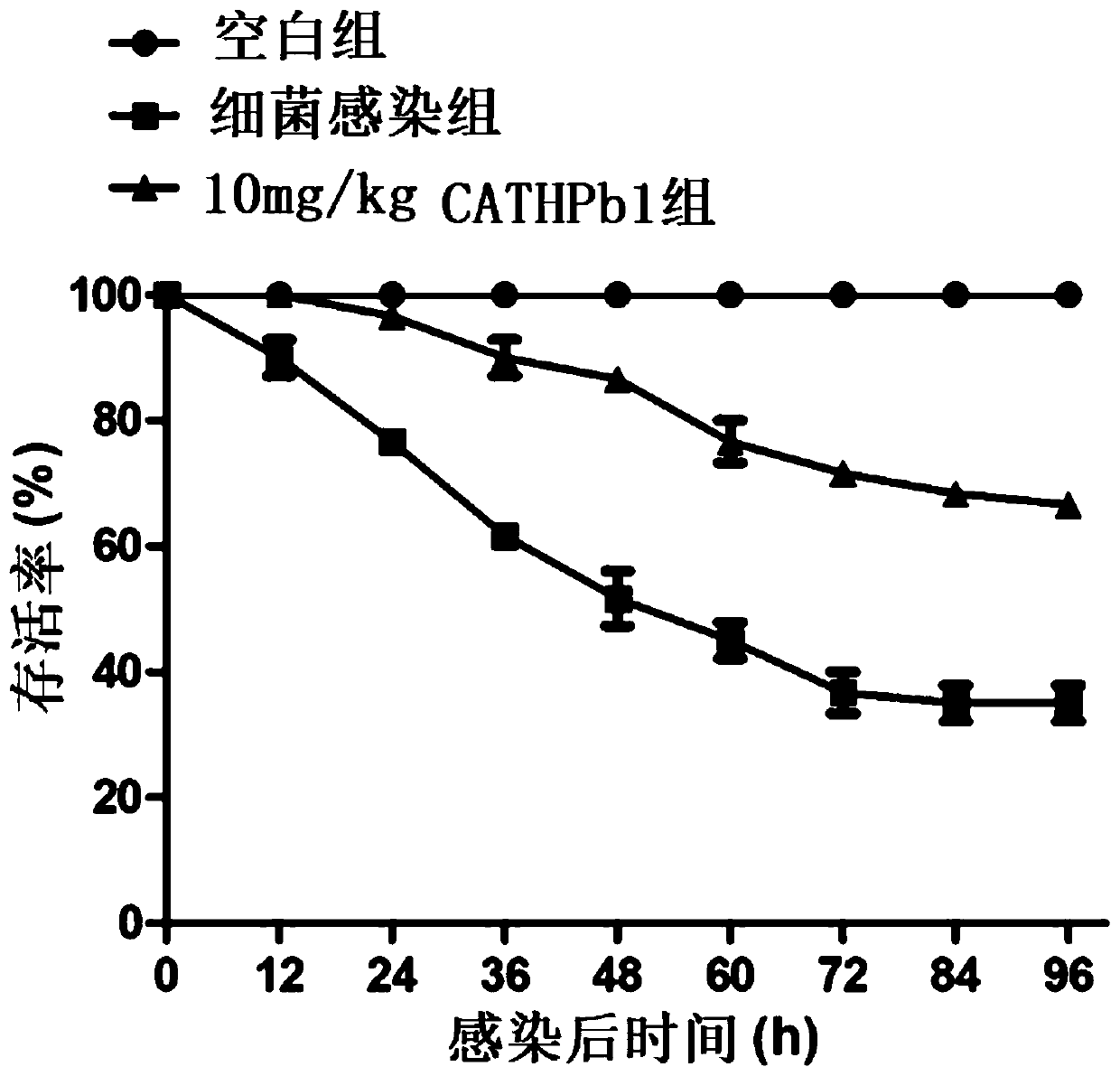
Applications of natural host defense peptide CATHPb1
A host defense peptide, a natural technology, applied in the field of peptide applications, can solve the problems of bacterial drug resistance drug residues, unscientific and other problems, and achieve the effects of broad-spectrum and high-efficiency antibacterial effects, improving resistance, and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of CATHPb1
[0026] (1) Using an automatic peptide synthesizer (433A, Applied Biosystems) to synthesize the complete sequence of CATHPb1 derived from the reptile Burmese python, and desalting and purifying it by HPLC reverse-phase column chromatography.
[0027] (2) The molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
[0028] (3), the purity of the purified CATHPb1 is identified by high performance liquid chromatography (HPLC), the molecular weight is determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), the isoelectric point is determined by isoelectric focusing electrophoresis, and it is determined by an automatic amino acid sequencer Amino acid sequence structure.
[0029] The amino acid sequence of the natural host defense peptide CATHPb1 of the present invention is shown in SEQ ID No.1. It consists of 31 amino acids,...
Embodiment 2
[0030] Example 2 Detection of antibacterial activity of CATHPb1 on common aquatic pathogens
[0031] (1), respectively pick the test strains (aquatic pathogenic bacteria) preserved on the slant and smear them evenly on the nutrient broth solid medium (nutrient broth medium, British OXOID company) flat plate, the 0.5cm diameter through sterilization Place the filter paper on the surface of the culture medium, drop 10 μL of 2 mg / mL CATHPb1 sample solution dissolved in sterilized deionized water, incubate upside down at 37°C for 18-20 hours, and observe whether the inhibition zone is formed or not. If the sample has antibacterial activity, a clear and transparent bacteriostatic zone will be formed around the filter paper, and the larger the bacteriostatic zone, the stronger the antibacterial activity of the sample.
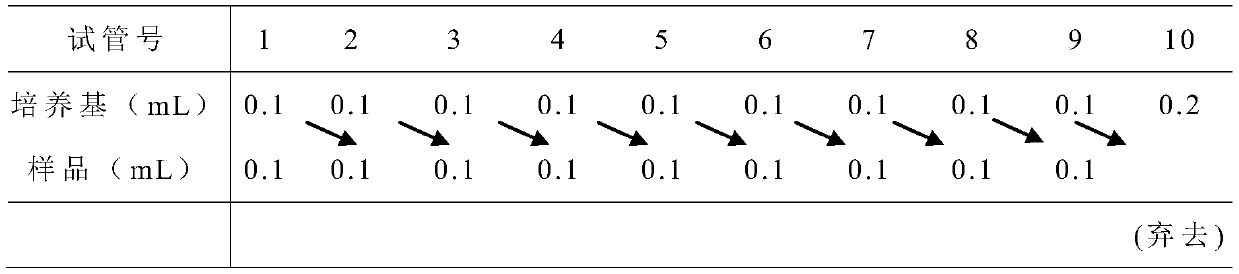
[0032] (2), CATHPb1 minimum inhibitory concentration (Minimum Inhibitory Concentration) determination (2-fold dilution method):
[0033] Select the strains with the...
Embodiment 3
[0042] Example 3 Determination of CATHPb1 sterilization speed
[0043] Vibrio parahaemolyticus was cultured at 37°C for 12 hours with NB liquid medium (OXOID, UK), and then diluted to 10 with fresh NB liquid medium. 6 CFU / mL bacterial suspension. The CATHPb1 sample dissolved in sterilized deionized water was added to the bacterial suspension so that the final concentration was 5×MIC. Place the bacterial solution added with the CATHPb1 sample in a 37°C incubator for shaking culture, take 50 μL of the bacterial solution to dilute 1000 times at 0, 15, 30, 60, 120 and 180 minutes respectively, and then take 50 μL of the diluted bacterial solution and spread it on the NB Count the colonies on the solid medium plate after culturing overnight in a 37°C incubator. In this experiment, neomycin sulfate was used as a positive control, and sterilized deionized water was used as a negative control.
[0044] The results are shown in Table 3, CATHPb1 has a rapid bactericidal speed to Vibr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
| Mic value | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com