Antibacterial peptide MS-CATH from Miniopterus fuliginosus and application thereof
A technology of MS-CATH and antimicrobial peptides, applied in the field of biomedicine, can solve problems such as cell death, damage to cell membrane integrity, dissolution of cell contents, etc., and achieve low hemolytic activity, broad-spectrum high-efficiency antibacterial effect, and rapid bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Discovery of Natural Antimicrobial Peptide MS-CATH
[0018] 1) Extraction of total RNA from long-winged bat lung tissue:
[0019] ① Take 100 mg of long-winged bat lung tissue, put it into a mortar and add liquid nitrogen to grind it into powder, transfer it to an EP tube, add 1 ml of total RNA extraction buffer (Trizol, product of Life, USA), mix well, and then place in Centrifuge at 12000rpm for 10min at 4°C.
[0020] ② Centrifuge to take the supernatant, add 0.2ml chloroform solution, mix vigorously, let stand at room temperature for 10 minutes, then centrifuge at 4°C, 12000rpm for 10 minutes, and discard the precipitate.
[0021] ③ Add an equal volume of isopropanol to the supernatant, leave it at room temperature for 10 minutes, centrifuge at 4°C, 12,000 rpm for 10 minutes, collect the precipitate, wash it once with 75% (V / V) ethanol, and dry it. The precipitate at the bottom of the tube is Wuyi Turbulence Total RNA from frog skin.
[0022] 2) Second-strand cDNA ...
Embodiment 2
[0037] Chemical Synthesis of Antimicrobial Peptide MS-CATH
[0038] (1) Synthesize the full sequence of MS-CATH with an automatic peptide synthesizer (433A, Applied Biosystems), and pass HPLC C 18 Desalting and purification by reverse phase column chromatography. (2) The molecular weight was determined by conventional matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF). (3) The purity of the purified MS-CATH was identified by high performance liquid chromatography (HPLC)>95%, and the isoelectric point determined by isoelectric focusing electrophoresis was 10.16. The amino acid sequence structure was determined to be consistent with natural MS-CATH by an automatic amino acid sequencer.
Embodiment 3
[0040] Detection of antibacterial activity of antimicrobial peptide MS-CATH
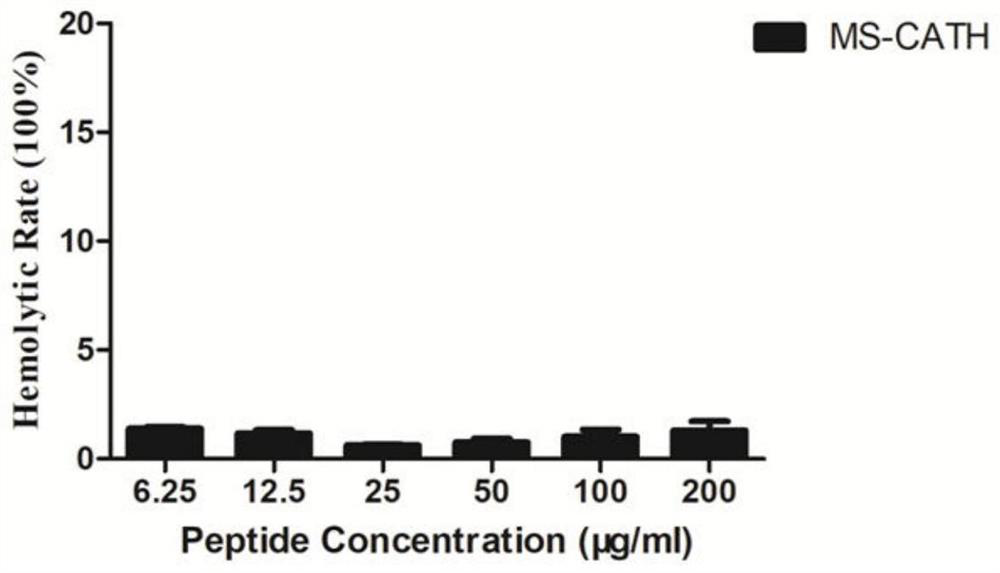
[0041] (1) Pick the test strains stored on the slope and evenly spread them on the MH solid medium (purchased from Qingdao Haibo Biotechnology Co., Ltd.) plate, and place the sterilized 0.5cm diameter filter paper on the medium On the surface, add 10 μl of 2 mg / ml MS-CATH sample solution dissolved in sterilized deionized water dropwise, incubate upside down at 37°C for 18-20 hours, and observe whether the inhibition zone is formed or not. If the sample has antibacterial activity, a clear and transparent bacteriostatic zone will be formed around the filter paper, and the larger the bacteriostatic zone, the stronger the antibacterial activity of the sample. The results showed that the antimicrobial peptide MS-CATH had antibacterial activity against the strains listed in Table 1.
[0042] (2) Determination of the Minimum Inhibitory Concentration (Minimum Inhibitory Concentration) by the 2-fold dilution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com