Corona Virus Disease 2019 (COVID-19) DNA vaccine and preparation method and application thereof
A technology of DNA vaccines and coronaviruses, applied in the field of vaccines, can solve problems such as the long process of vaccine development, achieve the effect of maintaining functionality and antigenicity, and increasing expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
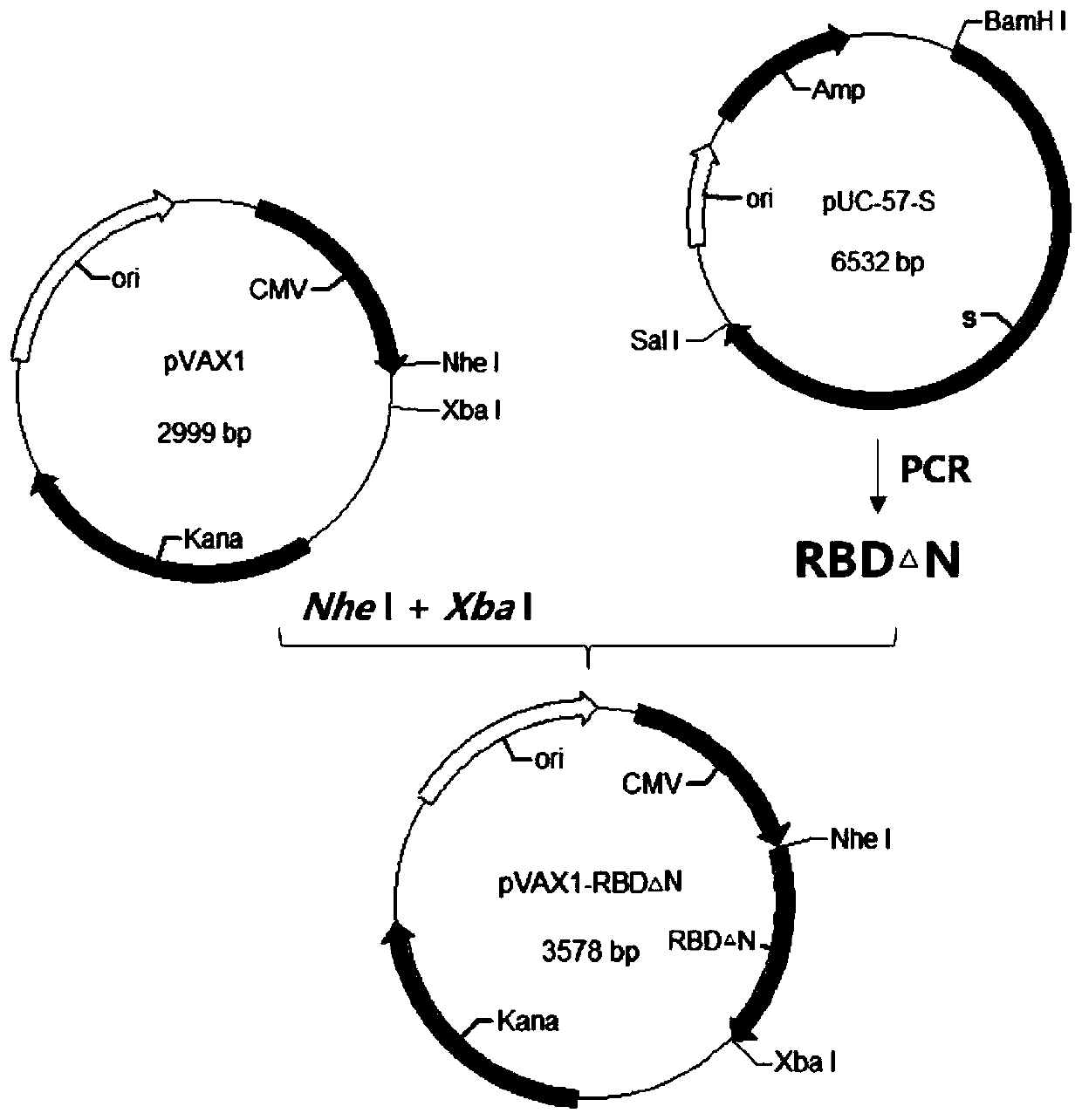
[0038] Example 1 Construction of plasmid pVAX1-RBD△N
[0039] (1) Acquisition of RBD△N gene
[0040] ①Template preparation
[0041] The commercialized novel coronavirus pneumonia virus S gene full-length plasmid pUC-57-S produced by Shanghai Shenggong Company was used as a template for PCR amplification.
[0042] ②Primer design
[0043]Using the primer design software, refer to the relevant sequences registered in Genbank, and design primers as shown in Table 1, that is, to amplify the RBD△N gene from pUC-57-S.
[0044] Table 1 Design primer sequence
[0045] Primer name sequence upstream primer GGGAGACCCAAGCTGGCTAGCGCCACCATGATTACAAACTTGTGCCCTTTTG downstream primer GGTTTAAACGGGCCCCTTAGATTAACCTGTGCCTGTTAAACCATT
[0046] ③PCR reaction
[0047] Use NEB company Q5 high-fidelity DNA polymerase to amplify RBD△N gene, add 5×Q5Buffer 10 μL, dNTP Mixture (2.5mM) 8 μL, upstream primer 1 μL, downstream primer 1 μL, plasmid 1 μL, Q5 enzyme 0.5 μL accordi...
Embodiment 2
[0051] Example 2 Identification of New Coronavirus DNA Recombinants
[0052] (1) Enzyme digestion and identification of recombinant DNA of the new coronavirus
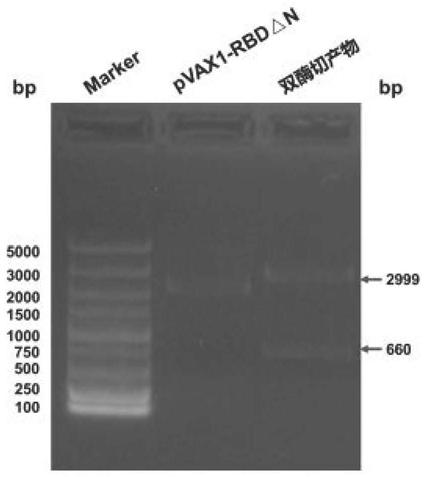
[0053] The ligation product was transformed into Escherichia coli competent cells, 200 μL was spread on LB agar plate, and cultured overnight at 37°C. Pick a single colony and inoculate it in 2 mL of LB culture medium containing corresponding antibiotics, and prepare a small amount of plasmid DNA. The recombinant plasmid was digested with restriction endonucleases Nhe I and Xba I, and analyzed by agarose gel electrophoresis. Select those whose digestion results are the same as expected for further identification, and those whose digestion results are exactly the same as expected are positive recombinant plasmids, such as figure 2 shown.
[0054] (2) New coronavirus DNA recombinant transfected cells
[0055] ① Cell count
[0056] Take 0.5mL of cell suspension, add 0.5mL of trypan blue solution, shake fully, place ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com