Recrystallization process method of nafamostat mesylate
A technology of nafamostat mesylate and a process method, applied in the field of recrystallization process of nafamostat mesylate, can solve the problems of increasing raw material loss and cost, unable to guarantee recrystallization effect, increasing the number of recrystallization times, etc. , to achieve the effect of improving purity, yield balance and purity balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
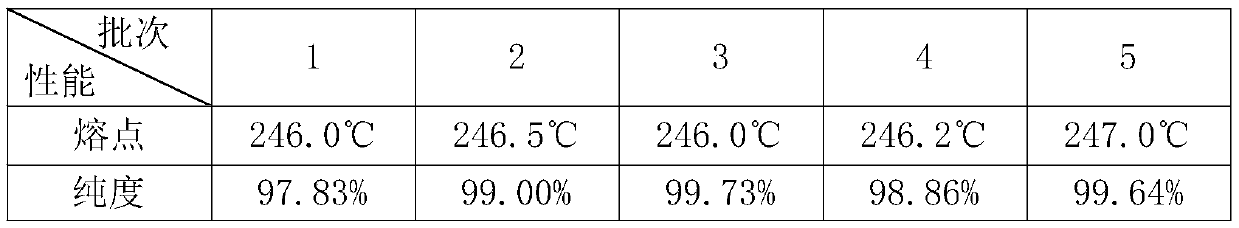
Embodiment 1
[0022] A. p-guanidinobenzoic acid hydrochloride: 12 parts, 6-amidine-2 naphthol methanesulfonate: 10 parts, DCC: 11 parts, DMAP1: 1 part, stirring continuously under ice bath for 24 hours, the whole reaction Protect from light, until the white solid is completely produced, filter, pour the filtrate into excess saturated sodium bicarbonate solution under stirring, and precipitate a brown powdery solid, wash with water, thoroughly wash to remove excess sodium salt, and dry to obtain nafamostat carbonate;
[0023] B. Suspend the nafamostat carbonate solid prepared in step A in 150mL methanol, add methanesulfonic acid dropwise under ice bath, until the solution is clarified, add 300mL ether, and separate out a yellow solid to obtain nafamostat mesylate Sestat crude;
[0024] C. For the first recrystallization, add 30mL of mixed solvent (ethanol: water = 1:1) and 8g of crude product into the beaker, and quickly put it into a 70°C water bath under rapid stirring. After the crude pro...
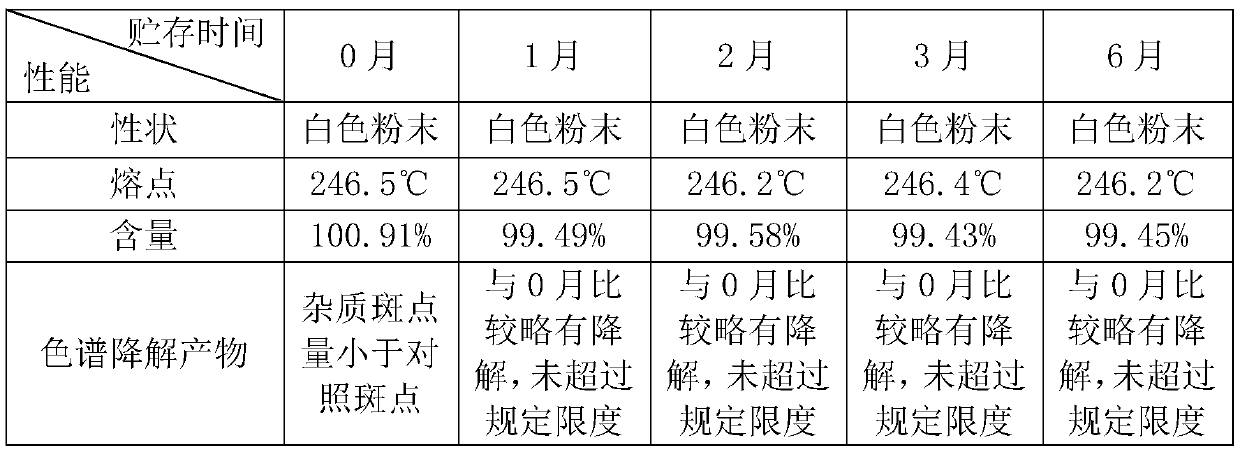
Embodiment 2
[0027] A. p-guanidinobenzoic acid hydrochloride: 12 parts, 6-amidine-2 naphthol methanesulfonate: 10 parts, DCC: 12 parts, DMAP1: 1 part, stirring continuously under ice bath for 48 hours, the whole reaction Protect from light, until the white solid is completely produced, filter, pour the filtrate into excess saturated sodium bicarbonate solution under stirring, and precipitate a brown powdery solid, wash with water, thoroughly wash to remove excess sodium salt, and dry to obtain nafamostat carbonate;
[0028] B. Suspend the nafamostat carbonate solid prepared in step A in 150mL methanol, add methanesulfonic acid dropwise under ice bath until the solution is clarified, add 500mL ether, separate out a yellow solid, and obtain nafamostat mesylate Sestat crude;
[0029] C. For the first recrystallization, add 30mL of mixed solvent (ethanol: water = 1:1) and 8g of crude product into the beaker, and quickly put it into an 80°C water bath under rapid stirring. A good hot filter fu...
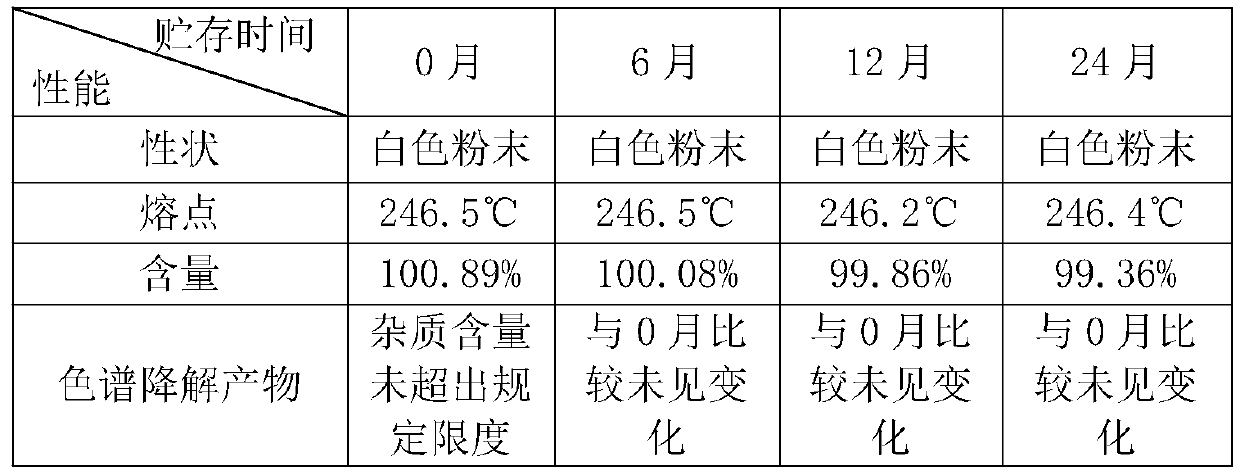
Embodiment 3
[0033] A. p-guanidinobenzoic acid hydrochloride: 11 parts, 6-amidine-2 naphthol methanesulfonate: 10 parts, DCC: 13 parts, DMAP1: 1 part, stirring continuously under ice bath for 72 hours, the whole reaction Protect from light; until the white solid is completely produced, filter, pour the filtrate into an excess saturated sodium bicarbonate solution under stirring, and separate out a brown powdery solid, wash with water, thoroughly wash to remove excess sodium salt, and dry to obtain nafamostat carbonate;
[0034] B. Suspend the nafamostat carbonate solid prepared in step A in 150mL methanol, add methanesulfonic acid dropwise under ice bath until the solution is clarified, add 700mL ether, and separate out a yellow solid to obtain nafamostat mesylate Sestat crude;
[0035]C. For the first recrystallization, add 30mL of mixed solvent (ethanol: water = 1:1) and 8g of crude product into the beaker, and quickly put it into a 90°C water bath under rapid stirring. A good hot filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com