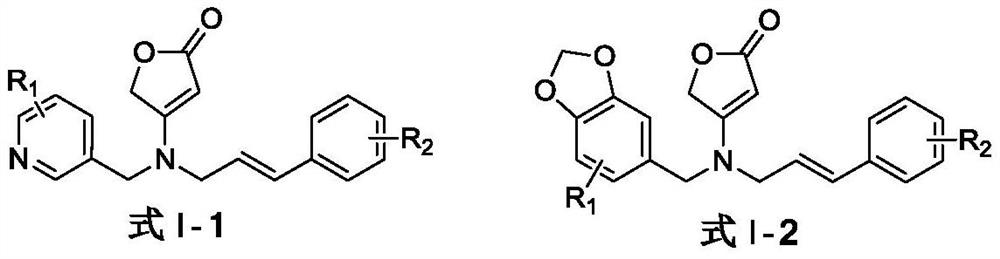
A compound containing natural butenolactone skeleton, its preparation and application
A technology of skeleton compound and butenolactone, which is applied in the field of agricultural pest control and agricultural pest control, can solve the problems that plant pathogenic fungi are prone to drug resistance and the effective use period of fungicides is shortened, and achieve good agricultural medicinal research value, Good inhibitory effect, broad-spectrum antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
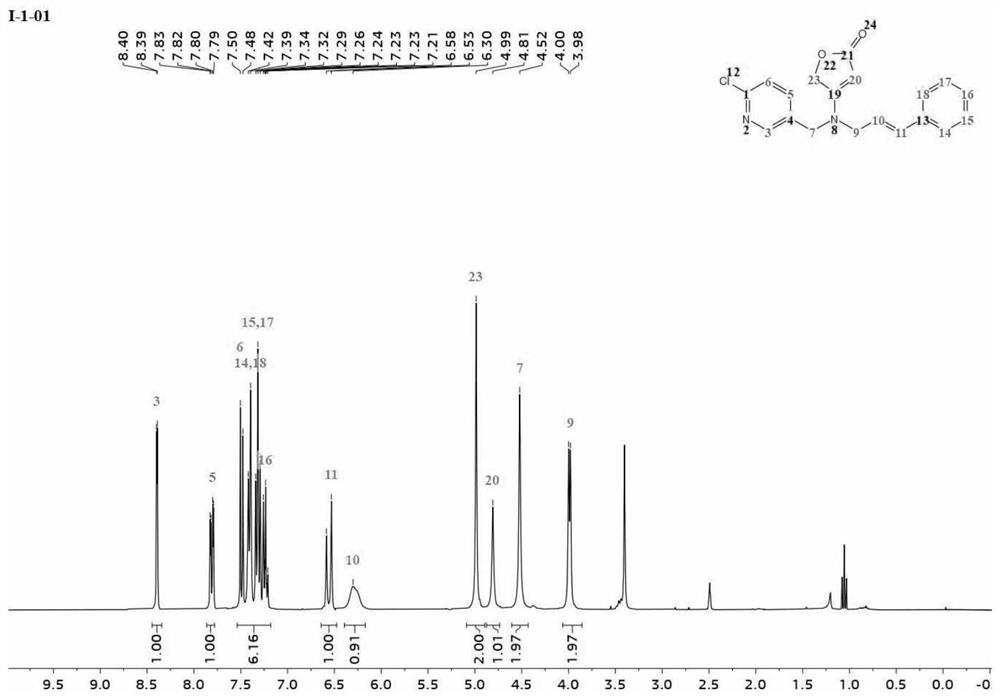
[0032] Embodiment 1: Preparation of butenolactone compound I-1-01 containing pyridine ring
[0033] Material 1 (0.13 mol) was dissolved in 125 mL of dimethylformamide (DMF), then material 2 (0.12 mol) was added dropwise to the above mixture and stirred at 35° C. for 8 h. After the reaction was complete, the solvent was depressurized. Water (45 mL) was added and extracted three times with 30 mL of toluene. The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 2-methoxy-2-oxoethylmethylmalonate 3. 0.1 mol of intermediate 3 was dissolved in 50 ml of pure methanol, and the reaction mixture was heated to 40°C. 30% sodium methoxide solution was added dropwise, refluxed for 3h, and a certain amount of white solid was separated. After the reaction, the mixture was cooled to 0°C, the precipitate was collected by filtration, washed with methanol, and dried to obtain 4-(methoxycarbonyl)-5-oxo-2,5-dihydrofuran-3-sodium oleate 4, which was col...
Embodiment 2
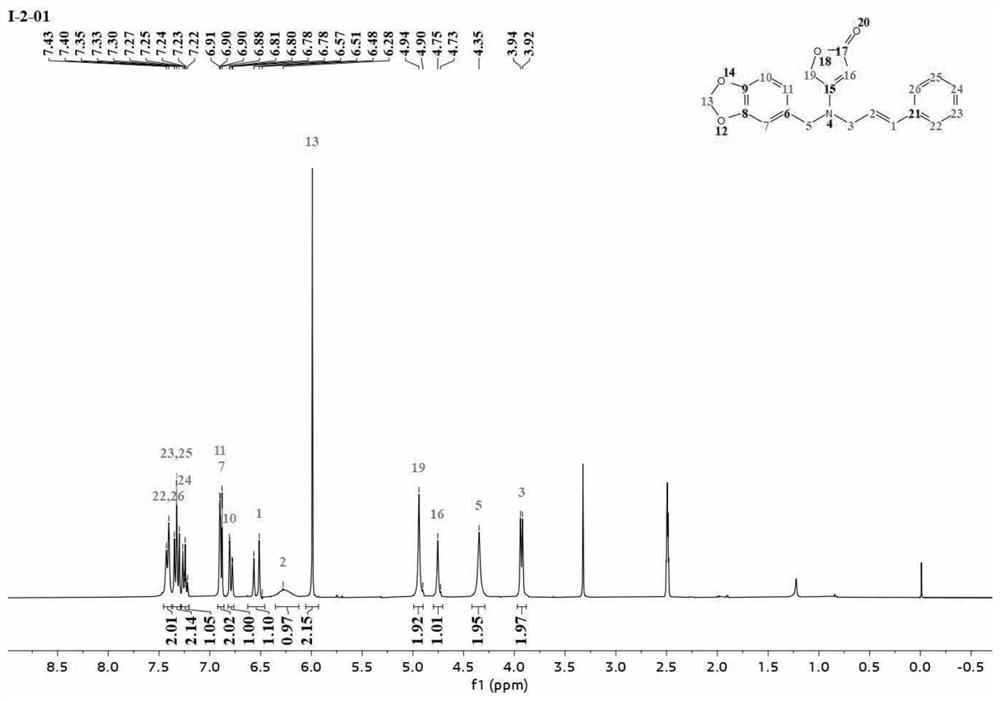
[0042] Example 2: Preparation of butenolactone compound I-2-01 containing piperonyl ring
[0043] Material 1 (0.13 mol) was dissolved in 125 mL of dimethylformamide (DMF), then material 2 (0.12 mol) was added dropwise to the above mixture and stirred at 35° C. for 8 h. After the reaction was complete, the solvent was depressurized. Water (45 mL) was added and extracted three times with 30 mL of toluene. The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 2-methoxy-2-oxoethylmethylmalonate 3. 0.1 mol of intermediate 3 was dissolved in 50 ml of pure methanol, and the reaction mixture was heated to 40°C. 30% sodium methoxide solution was added dropwise, refluxed for 3h, and a certain amount of white solid was separated. After the reaction, the mixture was cooled to 0°C, the precipitate was collected by filtration, washed with methanol, and dried to obtain 4-(methoxycarbonyl)-5-oxo-2,5-dihydrofuran-3-sodium oleate 4, which was colle...
Embodiment 3
[0052] Example 3: Determination of the activity of killing soybean aphids with butenolactone skeleton structure compounds
[0053] Weigh 2g of agar powder and 98g of water, heat it in a microwave oven, take it out after heating for 15 seconds, and heat it several times to make a transparent agar solution, pour it into a 12-well plate, and let it dry naturally for later use. Weigh 6mg sample in a 1.5mL centrifuge tube, dissolve it with 0.6mL DMSO, pour it into a 50mL small beaker with a 0.5mL pipette, and add 9.5mL TritonX-100 (5‰) aqueous solution to prepare a 500mg / L solution . Soybean leaves that were not exposed to any chemicals and insects were cultivated indoors, and the leaves of appropriate size were punched with a punch with a diameter of 15 mm, and immersed in a diluted solution containing chemicals for 15 seconds, and the leaves were naturally dried until the solvent evaporated After placing in a 12-well plate, turn the back of the leaf upside down, and place 20 soy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com