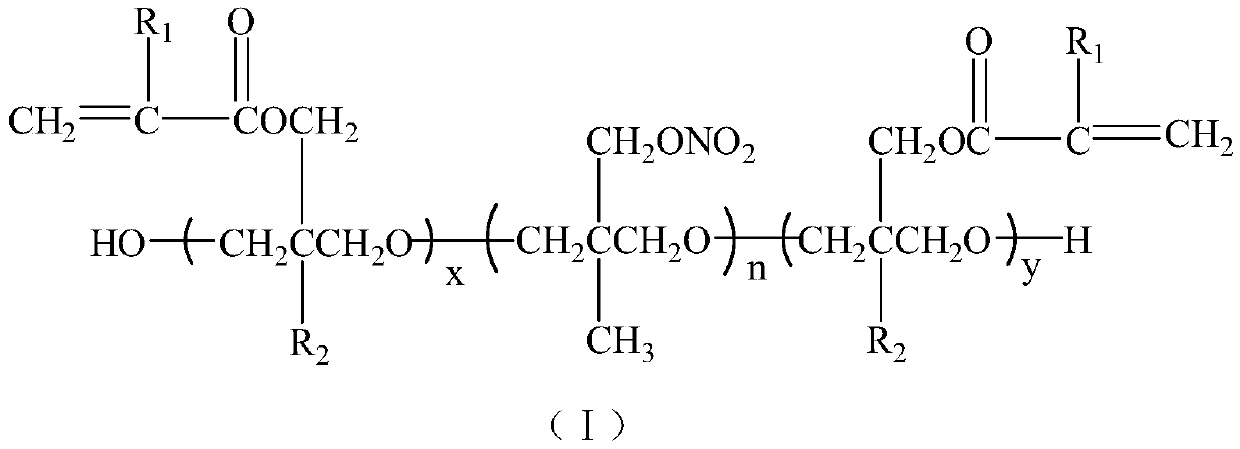
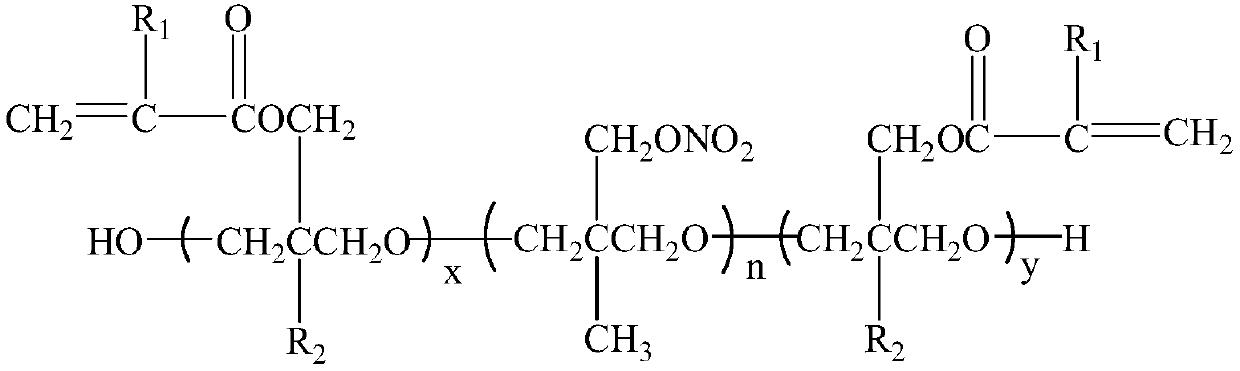
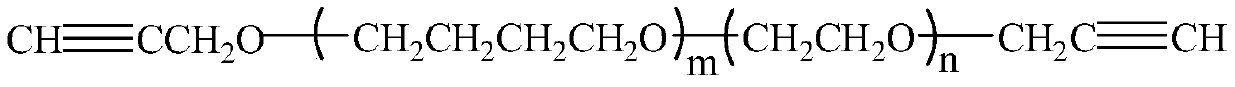Dual-cured nitrate polyether and synthesis method thereof
A nitrate ester and dual curing technology, applied in the direction of attacking equipment, compressed gas generation, etc., can solve the problems of low mechanical properties such as film tensile strength and elongation at break, and achieve moderate curing rate, wide adjustment range, high The effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] At room temperature, add 30g (10mmol) hydroxyl-terminated poly-3-nitrate methyl-3-methyloxetane and 60mL dichloromethane into the reaction flask, stir well, then add 1.42g (10mmol) trifluoro Boronium ether complex, reacted for 30min under stirring, cooled to 0°C, began to slowly add 3.68g (20mmol) of 3-methacryloyloxymethyl-3-ethyloxetane dropwise, and the dropwise addition was completed Afterwards, the reaction was continued for 24 hours, neutralized with aqueous sodium carbonate solution, washed with water until neutral, the oil phase was separated and dichloromethane was distilled off under reduced pressure to obtain a light yellow viscous liquid.
[0027] Structure Identification:
[0028] IR, ν max (cm -1 ): 3651 (-OH), 2941, 2863, 2804 (-CH 2 -, -CH 3 ), 1719 (-COO-), 1637 (C=C-), 1632, 1281, 869 (-ONO 2 ), 1114 (C-O-C).
[0029] 1 HNMR (CDCl 3 ,500MHz): δ6.13, 5.60, 4.39, 4.25, 3.23~3.30, 1.96, 1.76, 0.90.
[0030] 13 C NMR (CDCl 3 ,500MHz): δ167.9, 13...
Embodiment 2
[0034] At room temperature, add 30g (10mmol) hydroxyl-terminated poly-3-nitrate methyl-3-methyloxetane and 60mL dichloromethane into the reaction flask, stir well, then add 1.42g (10mmol) trifluoro Boronium ether complex, reacted for 30min under stirring, cooled to 0°C, began to slowly add 7.36g (40mmol) of 3-methacryloyloxymethyl-3-ethyloxetane dropwise, and the dropwise addition was completed Afterwards, the reaction was continued for 28 hours, neutralized with aqueous sodium carbonate solution, washed with water until neutral, the oil phase was separated and dichloromethane was distilled off under reduced pressure to obtain a light yellow viscous liquid.
[0035] Molecular weight and distribution: Mn=3675, Mw=5733, Mw / Mn=1.56.
Embodiment 3
[0037] At room temperature, add 40g (10mmol) hydroxyl-terminated poly-3-nitrate methyl-3-methyloxetane and 80mL dichloromethane into the reaction flask, stir well, then add 1.42g (10mmol) trifluoro Boronium ether complex, reacted for 30min under stirring, cooled to 0°C, began to slowly add 5.52g (30mmol) of 3-methacryloyloxymethyl-3-ethyloxetane dropwise, and the dropwise addition was completed Afterwards, the reaction was continued for 28 hours, neutralized with aqueous sodium carbonate solution, washed with water until neutral, the oil phase was separated and dichloromethane was distilled off under reduced pressure to obtain a light yellow viscous liquid.
[0038] Molecular weight and distribution: Mn=4548, Mw=7186, Mw / Mn=1.58.
[0039] Dual curing type nitrate ester polyether application performance of the present invention
[0040] (1) Miscibility and reactivity evaluation with curing agent
[0041]Ethylene glycol diazide acetate (EGBAA), 1,5-diazido-3-nitroazapentane (D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com