Application of decitabine in preparation of medicine for treating inflammatory bowel diseases
A technology for inflammatory bowel disease and decitabine, applied in the field of preparing medicines for the treatment of inflammatory bowel disease, decitabine can solve problems such as high price, achieve low cost, high efficiency and remission rate, and promote expression. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
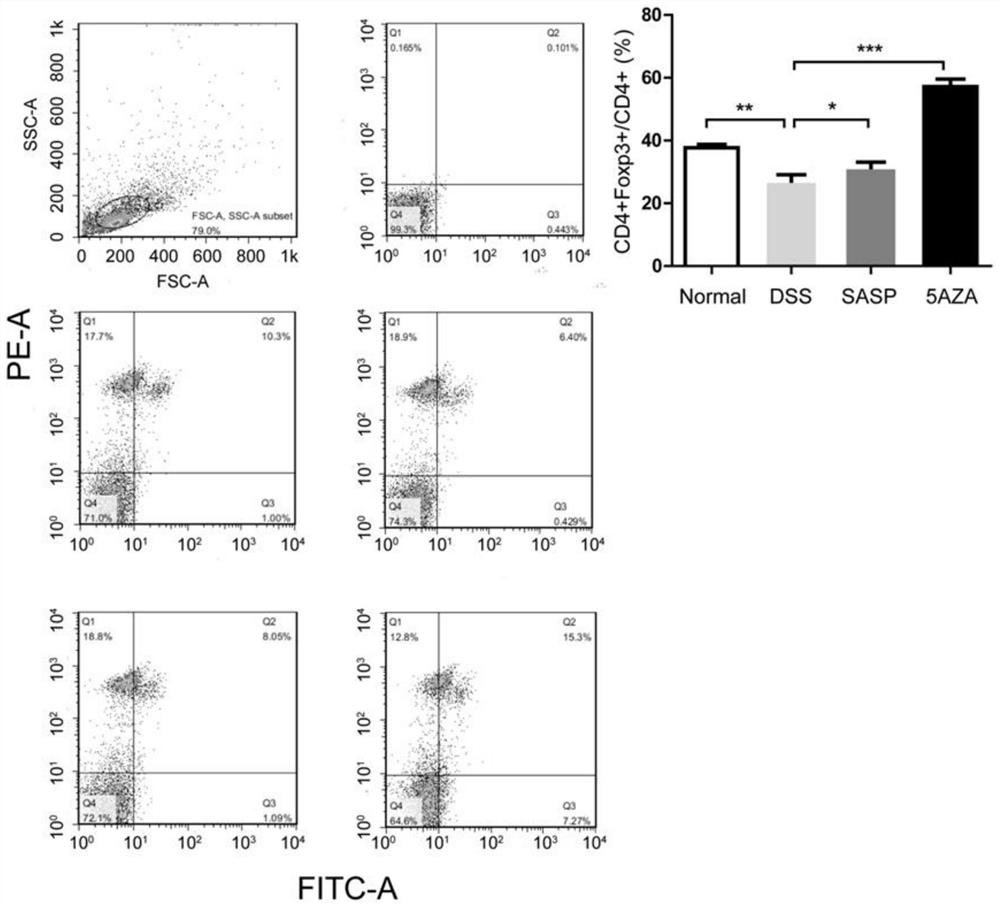
[0020] Cut out the mouse spleen, grind it with PBS as the homogenate medium, filter it through a 70um nylon mesh, centrifuge at 1500rpm for 5min, remove the supernatant, add 2ml 0.83% NH to the precipitate 4 CL to lyse red blood cells, add 15ml PBS after 2min to stop the lysis, centrifuge at 1500rpm for 5min, remove the supernatant, add 1ml PBS to the pellet, count, adjust the cell density to 10 6 / ml, added to 24-well plate, 1ml / wall. Add corresponding concentrations of anti-CD3 antibody (0.5 μg / ml) and anti-CD28 antibody (1 μg / ml), set up three duplicate wells, and set up PBS negative control wells, in 5% CO 2 , Incubate at 37°C for 72 hours, wash twice with PBS, add 0.05μg / μl APC-Ratanti-mouse CD4 antibody 50μl, stain in the dark at 4°C for 30min, wash twice with PBS, add 1 ml 3:1 diluted permeabilization fixation Add 2 ml permeabilization solution, centrifuge and wash twice; resuspend to 100 μl, add PE-Rat anti-mouse Foxp3 antibody, incubate at 4 ℃ in the dark for 1 h, ad...
Embodiment 2
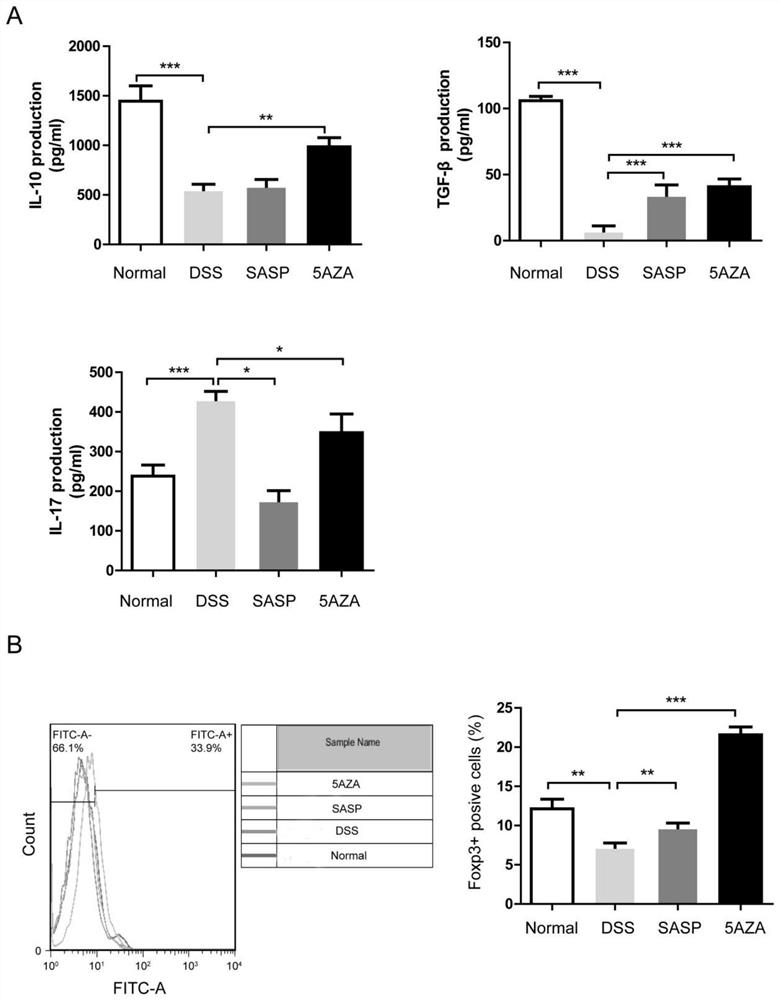
[0023] ELISA method was used to determine the changes of TH17 / Treg related cytokines IL-17, TGF-β, and IL-10 in mouse colon tissue: the colon tissue was washed on ice PBS, and about 10 mg of colon tissue was weighed after drying with filter paper , add 1 mL PBS (pH: 6.0, containing 1 μg aprotinin and leupeptin pepstatin A), cut it with ophthalmic scissors, put it into a homogenizer for homogenization, and centrifuge at 12000 r / min at 4 °C After 20 min, the supernatant was taken, and the concentrations of IL-17, TGF-β and IL-10 were detected according to the instructions of the ELISA kit.
[0024] experiment result shows( figure 2 ): Decitabine (5AZA) increases the content of regulatory T cell-related cytokines in the colon tissue of mice with inflammatory bowel disease, and the effect is better than that of sulfasalazine (SASP).
Embodiment 3
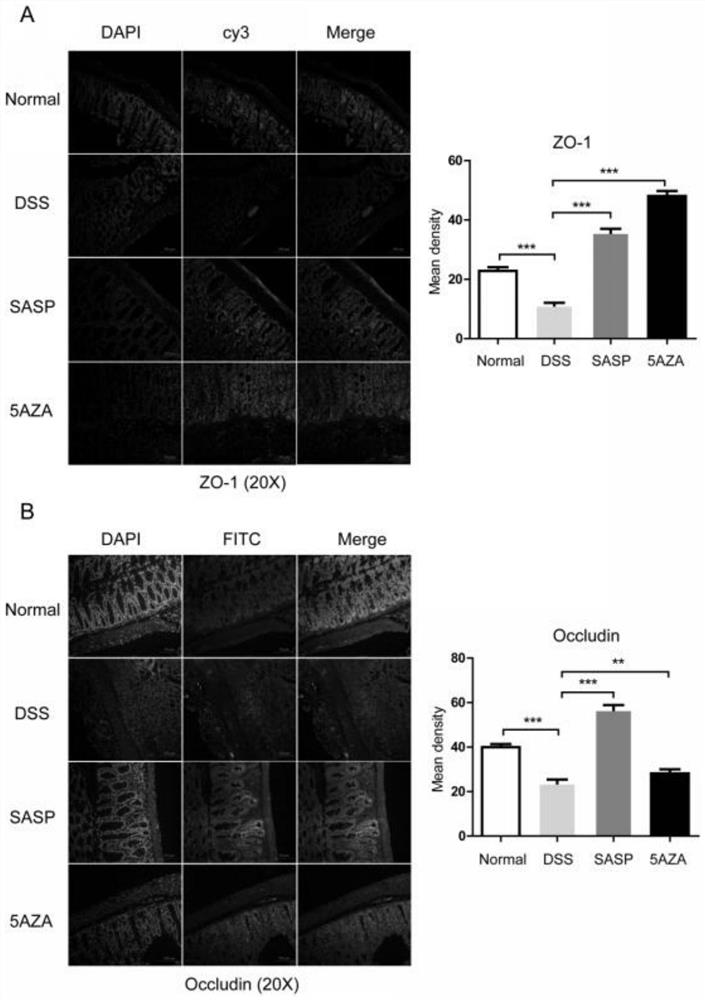
[0026] Immunofluorescence localization of tight junction protein expression: Fresh mouse colon tissue was washed with PBS, embedded in tissue embedding medium (Sakura, USA), and stored at -80°C. Frozen tissues were sliced at 10 μm and incubated in 10 mmol / L sodium citrate solution at 95°C for 5 min, then cooled to room temperature. Incubate with PBS containing 5% bovine serum albumin and 5% fetal bovine serum for 30 min at room temperature to eliminate non-specific background. The above sections were incubated with tight junction protein ZO-1 and Occludin primary antibodies overnight at 4°C, stained with fluorescein isothiocyanate-labeled phalloidin at room temperature for 1 h, and then incubated with corresponding fluorescein-labeled secondary antibodies Afterwards, the protein localization was observed under a confocal laser microscope.
[0027] experiment result shows( image 3 ): Decitabine (5AZA) improves the expression of tight junction protein in the colon tissue of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com