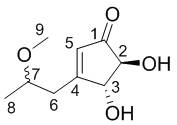
Preparation method of two aspergillus terreus ketone compounds and application of aspergillus terreus ketone compounds as antitumor drugs
A technology of terrein and compounds, applied in the field of medicine, to achieve the effects of inhibiting proliferation, inducing cell apoptosis, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
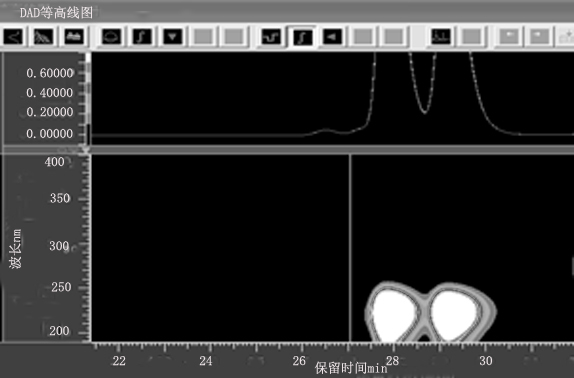
[0022] Separation and purification of formula I compound of the present invention
[0023] (1) Fermentation of mutant strains
[0024] CGMCC no. 19630 Aspergillus terreus mutant strain was inserted into seawater oligotrophic starch medium, and cultured on a shaker at 28°C and 150 rpm for 7 days.
[0025] (2) Extraction and separation of compounds
[0026] After the fermentation, the fermentation culture obtained in step (1) was filtered, and the bacterial liquid was extracted 3 times with ethyl acetate; the mycelium was soaked in dichloromethane / methanol (2:1), concentrated, and washed Extracted 3 times, combined extracts, concentrated under reduced pressure to obtain crude extract. The extract was first subjected to rapid decompression normal phase silica gel column chromatography, respectively with petroleum ether / ethyl acetate 10:1, 6:1,3:1, 1:1 and ethyl acetate / methanol 1:0, 9: 1 mobile phase gradient elution, a total of six components (Fr.1−Fr.6). Fr.5 was further se...
Embodiment 2
[0033] Cytotoxic activity test of formula I compound of the present invention
[0034] According to the literature method, the cytotoxic activity of the compound of formula I was tested by MTT or SRB method. The tested cell lines include human lung cancer cell A549, human cervical cancer cell Hela, human liver cancer cell Hep G2, human breast cancer cell MCF-7, and human colon cancer cell HCT-116. Doxorubicin was used as a positive control. Observe the Hoechst staining results, repeat the experiment three times, and calculate the IC of the compound on tumor cells 50 value.
[0035] The results showed that the compound of formula Ⅰ had a good inhibitory effect on Hep G2, and its IC 50 The values are 376 nM and 697nM respectively, and the Hoechst staining results are shown in the attached figure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com