Mouse CT26 colorectal cancer therapeutic tumor polypeptide vaccine preparation and preparation metheod of vaccine preparation
A tumor peptide vaccine, CT26 technology, applied in the direction of anti-tumor drugs, chemical instruments and methods, peptides, etc., can solve the problem of general anti-tumor immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
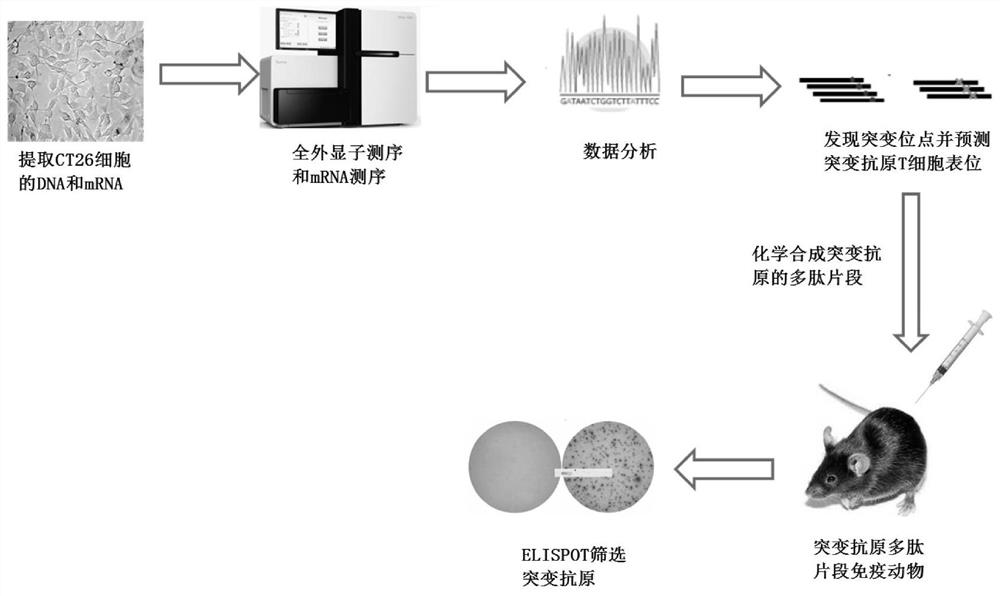
[0031] Example 1: Whole exome sequencing and mRNA gene expression sequencing.
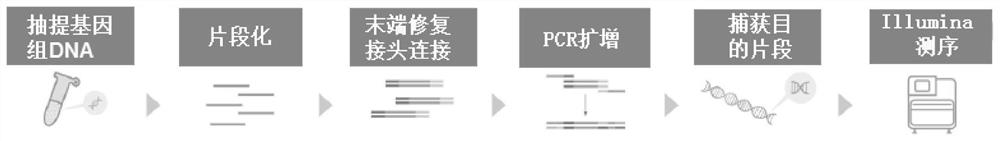
[0032] The CT26 tumor cell DNA was randomly broken into 150-200bp with an ultrasonic disruptor. After the magnetic beads were purified, the product was repaired and flattened by the end, and an A base was added to the 3 end to form a sticky end. Then, connect with a linker containing a specific barcode sequence. Magnetic bead screening removes incompletely connected products and self-connected products. Use universal primers complementary to the adapter sequence to perform PCR amplification to form a sequencing library. Then use Agilent probes to hybridize in hybridization buffer, use magnetic beads to capture the hybridized target fragments and separate and purify them. PCR amplifies the captured DNA fragments and purifies the PCR products to obtain a sequencing library. Qubit detects library concentration and Agilent 2100Bioanalyzer detects library fragment length. Finally, Illumina was used for...
Embodiment 2
[0033] Example 2: Prediction of tumor neoantigens.
[0034] Analyzing the data obtained by whole exome sequencing, a total of 1259 mutant peptide fragments were found in CT26 cells (Table 1). We used two tools, NetMHC and NetMHCpan, to predict the CD8 T cell epitope of these 1259 mutant peptide sequences. We scored the neoantigen mutation sequence according to the algorithm of the analysis tool, and then selected the top 20 mutant sequences with scores. Synthesize peptide fragments by chemical synthesis.
[0035] Such as figure 2 As shown, the exons of tumor cells were sequenced and analyzed, combined with gene expression analysis, to predict the mutant polypeptides produced by the mutations, and then the software NetMHC and NetMHCpan were used to perform MHC I restricted binding analysis on the mutant polypeptides, and it was predicted that they could be Mutant polypeptide fragments processed and presented by antigen-presenting cells. Then, according to the software score and t...
Embodiment 3
[0038] Example 3: ELISOPT screening of immunogenic mutant antigens
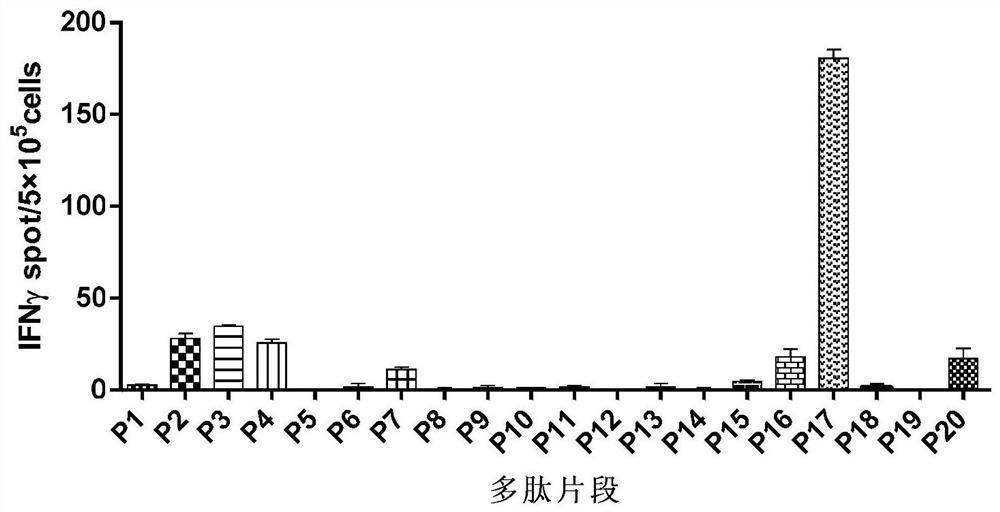
[0039] Mix 20 chemically synthesized mutant polypeptide fragments together with the adjuvant Poly:IC, and immunize BABL / C mice on the right back of the hind limbs. On the 7th day, a booster immunization will be carried out. After 14 days of immunization, take the small Mouse spleen, prepare splenic single cell suspension, and screen out the T cell epitope of the mutant antigen by ELISPOT technology. To produce IFN-γ spots greater than or equal to 10 / 5×10 5 A spleen cell is the positive standard. From the 20 mutant peptide fragments, a total of 7 polypeptide fragments with immunogenic mutant antigen CD8 T cell epitopes (see image 3 And Table 2).
[0040] Table 2 Polypeptide sequence in the present invention
[0041] SEQ ID NO:1P2Ufl1 KYQGLVVKQSV SEQ ID NO: 2P3Ufl1 VKYQGLVVKQSV SEQ ID NO: 3P4Ufl1 KYQGLVVKQSVK SEQ ID NO: 4P7 Rik DYVTGKMAV SEQ ID NO: 5P16 Nap14 SYIETLPKAIK SEQ ID NO: 6P17 Igf2r KYVFINVCHRV ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com