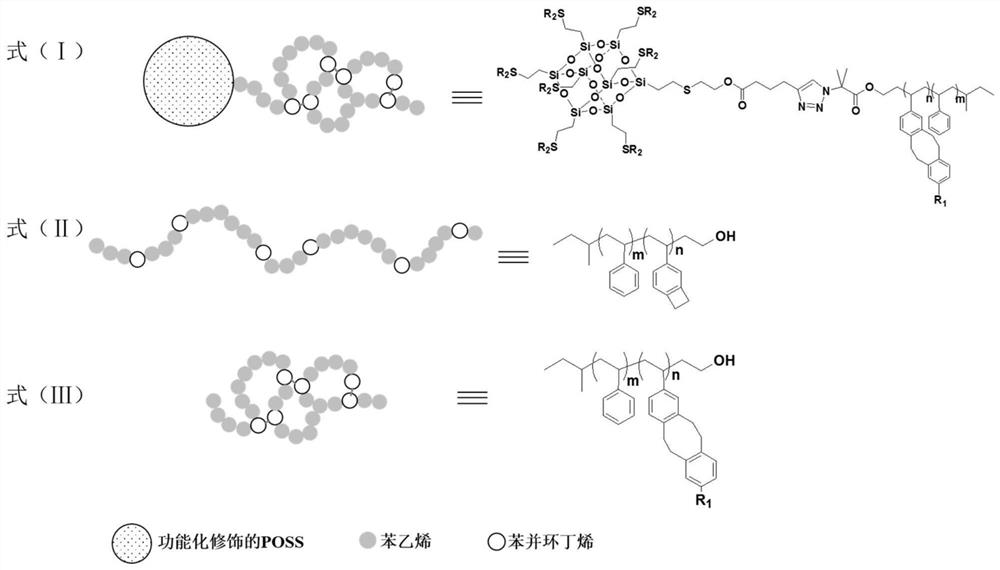
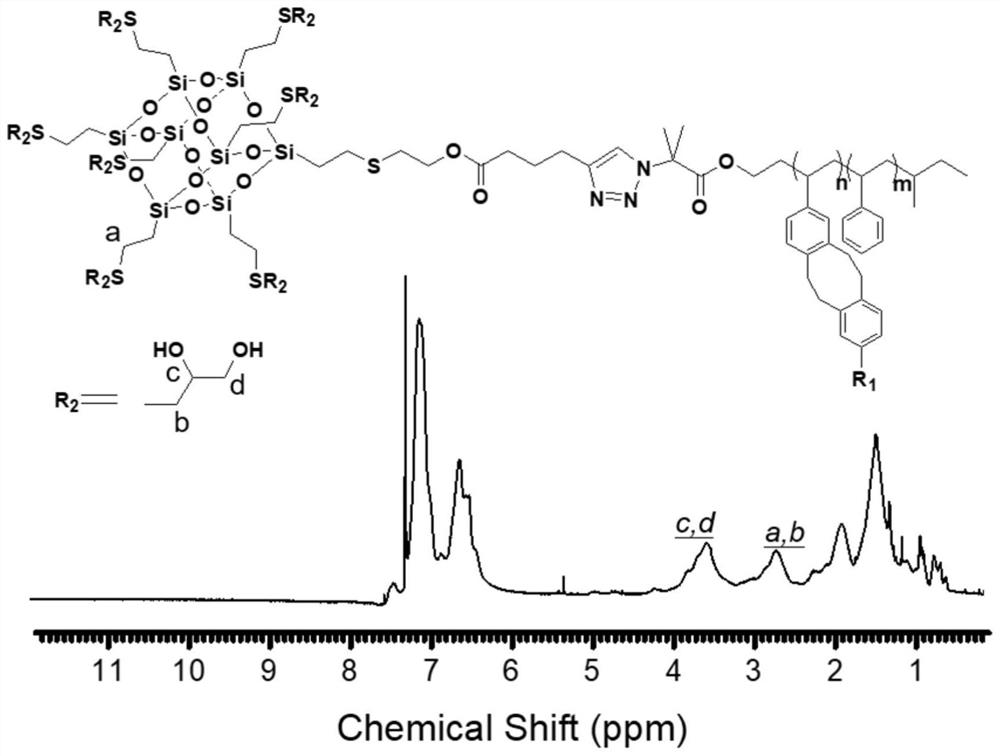
Giant surfactant and preparation method thereof
A surfactant and giant technology, applied in chemical instruments and methods, transportation and packaging, dissolution, etc., to achieve the effect of simple operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
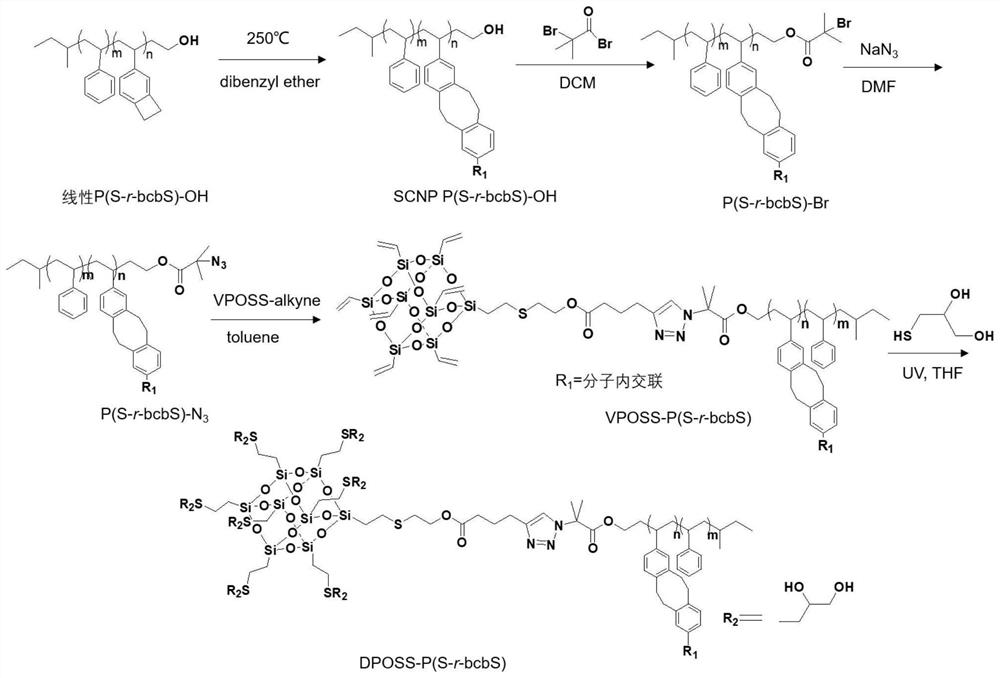
[0089] attached figure 2 It is a schematic diagram of the reaction process for the preparation of giant surfactants in Example 1. see figure 2 , this example prepares the method for giant surfactant, comprises the following steps:
[0090] Step 1: The raw material is linear poly(styrene-r-benzocyclobutene) (P(S-r-bcbS)-OH) with hydroxyl groups, the relative molecular mass is M=2600g / mol, m:n=9:1 . Add 250mL of dibenzyl ether into a 500mL three-neck round bottom flask, heat to 250°C while stirring, and fully dissolve 200mg (0.077mmol) of P(S-r-bcbS)-OH with 40mL of dibenzyl ether, and use a syringe pump to The solution was added dropwise into the heated dibenzyl ether at a speed of 150 μL / min. The whole process was vigorously stirred to ensure that the liquid was fully mixed. After the dropwise addition, the reactants were heated at 250° C. for 2 h. After the reaction was completed, all the dibenzyl ether solvent was removed by distillation under reduced pressure. The p...
Embodiment 2
[0097] This example prepares the method for giant surfactant, comprises the following steps:
[0098] Step 1: The raw material is linear poly(styrene-r-benzocyclobutene) (P(S-r-bcbS)-OH) with hydroxyl groups, the relative molecular mass is M=4800g / mol, m:n=2.3:1 . Add 250mL of dibenzyl ether into a 500mL three-necked round-bottomed flask, heat to 250°C while stirring, and fully dissolve 250mg (0.052mmol) of P(S-r-bcbS)-OH with 40mL of dibenzyl ether, and use a syringe pump Add the solution dropwise to the heated dibenzyl ether at a rate of 200 μL / min, and stir vigorously during the whole process to ensure that the liquid is fully mixed. After the dropwise addition, continue to heat the reactant at 250° C. for 2 h. After the reaction was completed, dibenzyl ether solvent was removed by distillation under reduced pressure, the product was dissolved in 1.5 mL of dichloromethane, dropped into 40 mL of methanol for precipitation, the liquid was removed by suction filtration, and t...
Embodiment 3
[0104] This example prepares the method for giant surfactant, comprises the following steps:
[0105]Step 1: The raw material is linear poly(styrene-r-benzocyclobutene) (P(S-r-bcbS)-OH) with hydroxyl groups, the relative molecular mass is M=6400g / mol, m:n=9:1 . Add 250mL of dibenzyl ether into a 500mL three-neck round-bottomed flask, heat to 250°C while stirring, and fully dissolve 250mg of P(S-r-bcbS)-OH with 40mL of dibenzyl ether, and use a syringe pump at 220μL / min The solution was added dropwise to the heated dibenzyl ether at a high speed, and the whole process was vigorously stirred to ensure that the liquid was fully mixed. After the dropwise addition, the reactant was continued to be heated at 250°C for 2 hours. After the reaction was completed, dibenzyl ether solvent was removed by distillation under reduced pressure, the product was dissolved in 1.5 mL of dichloromethane, dropped into 40 mL of methanol for precipitation, the liquid was filtered out by suction, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com