Application of ethanone compound in preparation of medicine for treating inflammation
A technology of acetone and drugs, applied in the field of PI3Kγ-specific inhibitors, can solve problems such as limited effects of drugs for inflammatory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
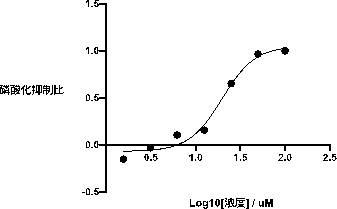
[0025] Example 1: The specific inhibitory effect of compounds on PI3Kγ
[0026] In vitro enzymatic experiments, using the broad-spectrum inhibitor PI-103 as a control, using the ADP-Glo kit to detect the compound 1-(5-(3-((4-chlorobenzyl)amino)-2-hydroxypropoxy)- Inhibitory activity of 2-methyl-1-(p-tolyl)-1hydro-indol-3-yl)ethanone on PI3Kα, PI3Kβ, PI3Kδ, PI3Kγ, respectively.
[0027] PI3Kγ blank group: use a 384-well plate to set up 3 parallel experimental groups. Prepare 5 μL of PI3K reaction system, each well contains 2.5 μL of PI3Kγ protein, add 2.5 μL of reaction substrate PIP2 and ATP to start the reaction, incubate at room temperature for 1 hour, use 5 μL of MgCl 2 The reagent terminated the reaction, and after incubation for 2 hours, 10 μL of ADP-Glo detection reagent was added, and after incubation for 45 minutes, the fluorescence value of the reaction solution was detected by a microplate reader.
[0028]Use the same operation to prepare PI3Kα, PI3Kβ, PI3Kδ bl...
Embodiment 2
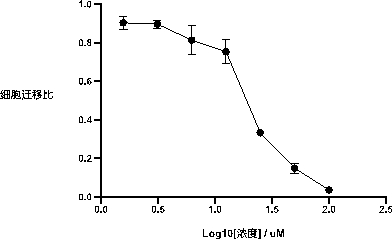
[0035] Example 2: Detection of specific inhibitory effect of compounds on PI3K pathway in inflammatory cells
[0036] Using RAW264.7 cells under the action of chemokine C5a as a cell model for specific activation of the PI3Kγ pathway, the compound 1-(5-(3-((4-chlorobenzyl)amino)-2-hydroxypropoxy)- Inhibitory effect of 2-methyl-1-(p-tolyl)-1hydro-indol-3-yl)ethanone on phosphorylation of the PI3K downstream effector molecule Akt.
[0037] The specific steps are as follows: the RAW264.7 cells in the logarithmic growth phase were plated on a 96-well plate at 200,000 per well, and 200 μL per well was used in serum-free DMEM medium. Place in a cell culture incubator at 37°C, 5% carbon dioxide overnight. Add the compound for 30 minutes before adding C5a stimulation, and the DMSO content does not exceed 5 / 1000. Then add 25 nM C5a for 3 minutes, remove the medium, add 50 μL of cell lysate to each well, place on ice, and then centrifuge at 3000 rpm at 4°C for 5 minutes. Perform an E...
Embodiment 3
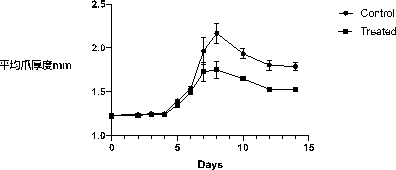
[0039] Example 3: Inhibitory Effect of Compounds on Inflammatory Cell Chemotaxis
[0040] Under the action of chemokines such as C5a, fMLP, CCL3, CXCL1–3 (KC), leukocytes, especially neutrophils and monocytes, stretch out pseudopodia and move along the low concentration gradient to the site of high concentration, Gather to the site where chemokines are produced to produce chemotaxis. Chemotaxis experiments were performed using Transwell technology. Motile cells will slowly migrate from low concentration to high concentration under the action of chemokines, that is, the cells inside the Transwell chamber will move to the outside of the chamber through the filter membrane.
[0041] The experiment was divided into control group and treatment group. First, put the inner side of the Transwell chamber into a 24-well plate, add DMEM serum-free medium to infiltrate the inner chamber, and take it out after 30 minutes. At the same time, the RAW264. (3-((4-chlorobenzyl)amino)-2-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com