Polymerized Schiff base type adsorption material and preparation thereof, and application in heavy metal adsorption
An adsorption material, Schiff base type technology, applied in adsorption water/sewage treatment, other chemical processes, water/sludge/sewage treatment, etc. It can solve the problems of unsatisfactory adsorption performance and poor stability, and achieve excellent adsorption effect. , The product properties are stable and easy to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
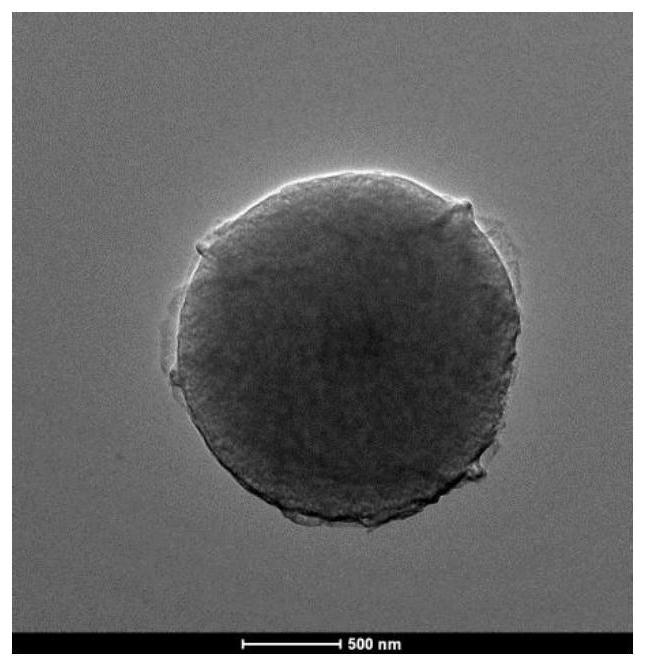
[0083] Take 1.057 mL of glutaraldehyde solution (50%) and add it into 15 mL of deionized water to prepare a glutaraldehyde solution, which contains 0.005 mol of glutaraldehyde. Dissolve 0.2704g of m-phenylenediamine (2.5mM) in 5mL of deionized water, and add the m-phenylenediamine solution into the prepared glutaraldehyde solution. 2 Stir mechanically under protective gas for 1 h. The suspension formed after the reaction was vacuum filtered, and the solid product obtained was washed three times with deionized water and absolute ethanol, and finally dried in vacuum at 50° C. for 12 h to obtain a sample and weigh it. The sample mass was 0.7096 g ( The yield is 92.1%). The TEM image of the sample is shown in figure 1 ; The particle size is 1 μm.
Embodiment 2
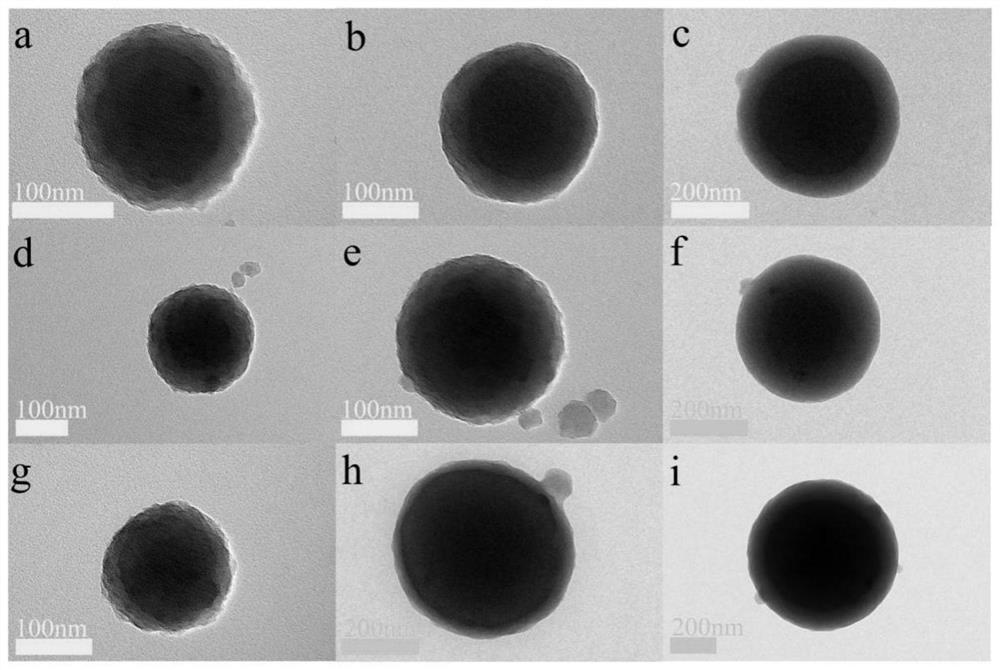
[0085] Take 528 μL of glutaraldehyde solution (50%) and add it into 15 mL of deionized water to prepare a glutaraldehyde solution, which contains 0.0025 mol of glutaraldehyde. Dissolve 0.2704g of m-phenylenediamine (2.5mM) in 5mL of deionized water, and add the m-phenylenediamine solution into the prepared glutaraldehyde solution. 2Stir mechanically under protective gas for 1 h. Add the suspension formed after the reaction into a closed polytetrafluoroethylene reactor, and react at 120° C. for 12 hours. The hydroheated product was vacuum filtered with a G5 sand core funnel, and the obtained solid product was washed three times with 70°C deionized water and absolute ethanol, and finally vacuum-dried at 50°C for 12 hours to obtain a sample and weigh it , sample quality 0.3377g (yield is 64.9%). The TEM image of the sample is shown in figure 2 a; particle size is 200nm; shell thickness is 20nm.
Embodiment 3
[0087] Take 1.057 mL of glutaraldehyde solution (50%) and add it into 15 mL of deionized water to prepare a glutaraldehyde solution, which contains 0.005 mol of glutaraldehyde. Dissolve 0.2704 g of m-phenylenediamine in 5 mL of deionized water, add the m-phenylenediamine solution into the prepared glutaraldehyde solution, and mechanically stir for 1 h under N2 as a protective gas. Add the suspension formed after the reaction into a closed polytetrafluoroethylene reactor and react at 120° C. for 12 hours. The hydroheated product was vacuum filtered with a G5 sand core funnel, and the obtained solid product was washed three times with 70°C deionized water and absolute ethanol, and finally vacuum-dried at 50°C for 12 hours to obtain a sample and weigh it , sample quality 0.650g (yield is 84.5%). The TEM image of the sample is shown in figure 2 b; particle size is 200nm; shell thickness is 50nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com