Small molecular compound for inhibiting activity of AKT and STAT3 and application of small molecular compound
A small molecule compound, active technology, applied in the direction of organic active ingredients, organic chemistry, drug combination, etc., to achieve the effect of inhibiting tumor cell proliferation and eliminating tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1, (E)-1-(3-(1-tosyl-1H-pyrrolo[2,3-b]pyridin-5-yl)-5,6-dihydropyridine-2(1H) preparation of
[0023] Step 1: Synthesis of 5-bromo-1-toluene-1H-pyrrolo[2,3-b]pyridine (f-1)
[0024]
[0025] 1.5 g of sodium hydride (60 mmol) was dissolved in 250 ml of tetrahydrofuran containing 10 g of 5-bromo-1H-pyrrolo[2,3-b]pyridine (50 mmol) at -50°C. Stir at -50 °C for 30 min, then add 12 g of p-toluenesulfonyl chloride (60 mmol) dissolved in 50 ml of tetrahydrofuran, and continue stirring at -50 °C for 1.5 h; extract and combine organic layers, concentrate in vacuo, and dry over sodium sulfate , purified by column chromatography to obtain 5-bromo-1-toluene-1H-pyrrolo[2,3-b]pyridine (ie compound f-1), 15 g of white solid, and the yield was 80%.
[0026] Step 2: Synthesis of (E)- ethyl 3-(1-toluenesulfonyl-1H-pyrrolo[2,3-b]pyridin-5-yl)acrylate (f-2)
[0027]
[0028] 5 g of compound f-1, 1.56 g of ethyl acrylate, 4.16 g of palladium acetate, and 4.32 g of tris(o-to...
Embodiment 2
[0038] Example 2, CCK-8 method to detect the activity of compound f-5 on glioblastoma cells
[0039] experimental method:
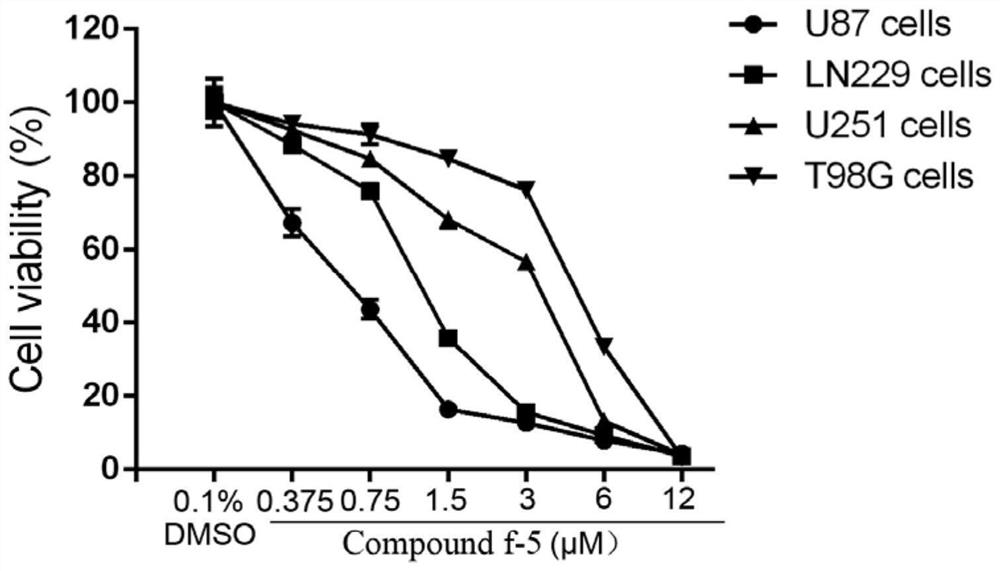
[0040]Glioblastoma cells were plated in triplicate on 96-well plates (3000 cells per well). After overnight culture, 0.1% DMSO was added to the control group, and 0.375 μM, 0.75 μM, 1.5 μM, 3 μM, 6 μM and 12 μM of compound f-5 were incubated for 72 hours. 10 μL of CCK-8 solution was added to each well, and after incubation for 2 hours, the absorbance at a wavelength of 450 nm was detected with a microplate reader.
[0041] Experimental results such as figure 1 It was shown that compound f-5 had growth inhibitory effects on the four glioblastoma cell lines in a concentration-dependent manner, and the IC50 values of the four cell lines ranged from 0.5 to 5.0 μM.
Embodiment 3
[0042] Embodiment 3, EdU incorporation test detects the inhibitory effect of compound f-5 on the proliferation of glioblastoma cells
[0043] experimental method:
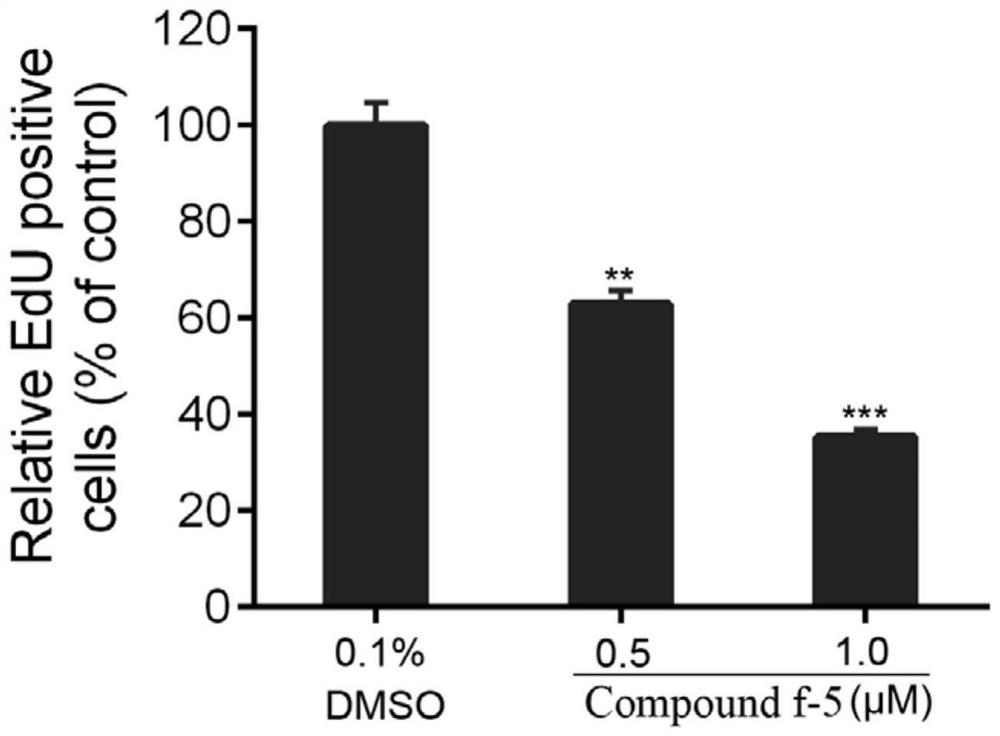
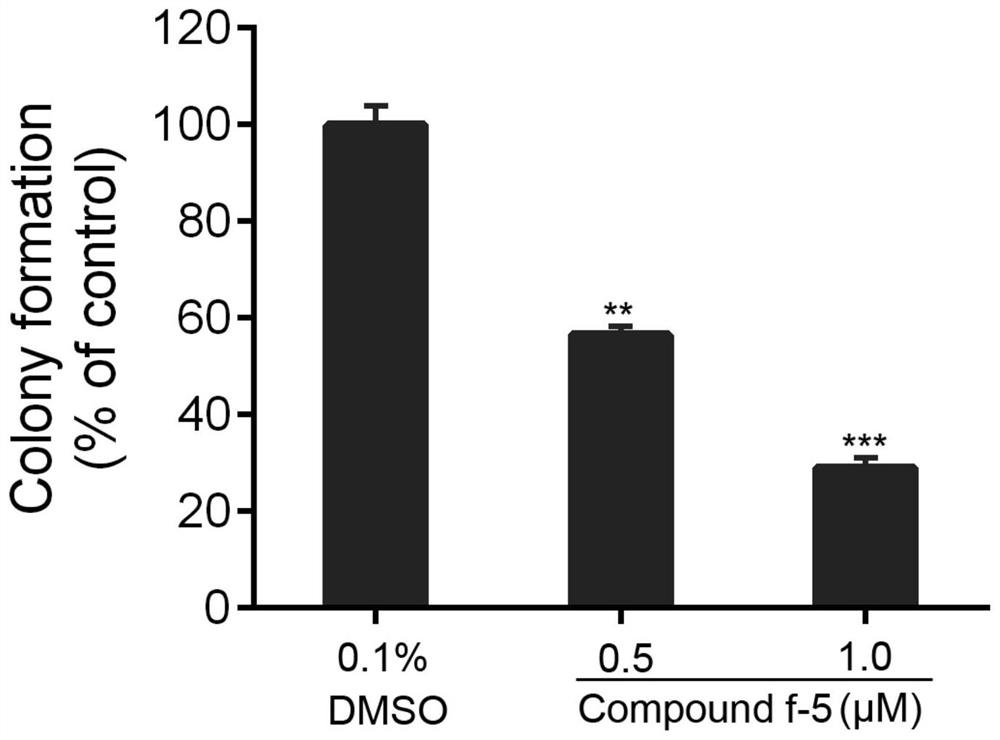
[0044] Cell proliferation was detected with the Cell-LightTM EdU Cell Proliferation Detection Kit according to the instructions. U87 cells were seeded in 96-well plates, and after the cells adhered to the wall, the cells were treated with 0.1% DMSO or 0.5 and 1.0 μM of compound f-5. After 24 hours, incubation was continued for 4 hours with 50 μM EdU, followed by fixation with 4% paraformaldehyde for 15 minutes and treatment with 0.5% Triton X-100 for 20 minutes. Cells were incubated with 1 × Apollo® reaction cocktail for 30 minutes and then stained with DPAI for 15 minutes. After washing with PBS three times, photographs were taken under a fluorescent inverted microscope, and the experiment was repeated three times.
[0045] Values represent relative percentages of EdU-positive cells in cells treated with diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com