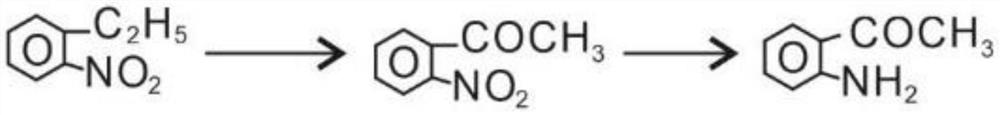Preparation method of o-aminoacetophenone
A technology of o-aminoacetophenone and nitroacetophenone, which is applied in the field of preparation of o-aminoacetophenone, can solve problems such as low yield of final products, and achieve the effects of low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A preparation method for o-aminoacetophenone, comprising the following steps:
[0023] (1) react oxidant potassium permanganate, acid catalyst, o-nitroethylbenzene in dilute sulfuric acid environment, after the reaction is finished, extract, wash, and dry to obtain o-nitroacetophenone;
[0024] (2) Add the o-nitroacetophenone and reduced metals obtained in step (1) to continuous reflux reaction in ammonium chloride aqueous solution. After the reaction is over, feed steam into the reaction kettle for distillation, and carry out the distilled solution. Separation can give o-aminoacetophenone.
[0025] It should be noted that: step (1) can only be reacted under acidic conditions. When reacting under alkaline environmental conditions, the product obtained will be affected. For example, when reacting under the alkaline conditions of sodium hydroxide, the obtained The product is o-nitrobenzoic acid. The oxidizing agent in the step (1) can only use potassium permanganate, an...
Embodiment 1
[0038]Preparation of o-nitroacetophenone: Add 210L of water into a 1000L enamel reaction kettle, start stirring, pass cooling water through the jacket of the kettle, slowly add 94kg of concentrated sulfuric acid dropwise, and add 3kg of glacial acetic acid, control the temperature at 30-40 degrees, and then Add 100kg o-nitroethylbenzene (662mol), and add 162kg potassium permanganate (1025mol) in small batches in 6 hours under rapid stirring, keep the temperature in the kettle no more than 50 degrees, and keep stirring for 2 hours after the potassium permanganate is added. hour, then add 10kg of industrial salt, stir and cool down to 30 degrees, let it stand for half an hour, after the manganese dioxide mud and the oil layer are clearly separated, the oil layer is sucked out under negative pressure; add 110kg of industrial benzene to the oily manganese mud left in the kettle, 10kg of salt, stir rapidly for 20 minutes, and then slowly stir for 10 minutes. After the manganese mud ...
Embodiment 2
[0041] Preparation of o-nitroacetophenone: Add 210L of water into a 1000L enamel reaction kettle, start stirring, pass cooling water through the jacket of the kettle, slowly add 94kg of concentrated sulfuric acid dropwise, and add 2.5kg of glacial acetic acid, control the temperature at 30-40 degrees, Then add 100kg o-nitroethylbenzene (662mol), and add 138kg potassium permanganate (873mol) in small batches in 6 hours under rapid stirring, keep the temperature in the kettle not exceeding 50 degrees, and keep stirring after the potassium permanganate is added. 2 hours, then add 10kg of industrial salt, stir and cool down to 30 degrees, let it stand for half an hour, after the manganese dioxide mud and the oil layer are clearly separated, suck out the oil layer under negative pressure; add 110kg of industrial benzene to the oily manganese mud left in the kettle, 10kg of industrial salt, stir rapidly for 20 minutes, then slowly stir for 10 minutes, after the manganese mud and benz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com