Preparation method of venlafaxine impurity E
An impurity and substance technology, applied in the field of preparation of venlafaxine impurity E, can solve the problems of waste water, solid waste, complicated treatment, low purity and yield, etc., and achieves convenient industrial production, simple post-processing, and product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
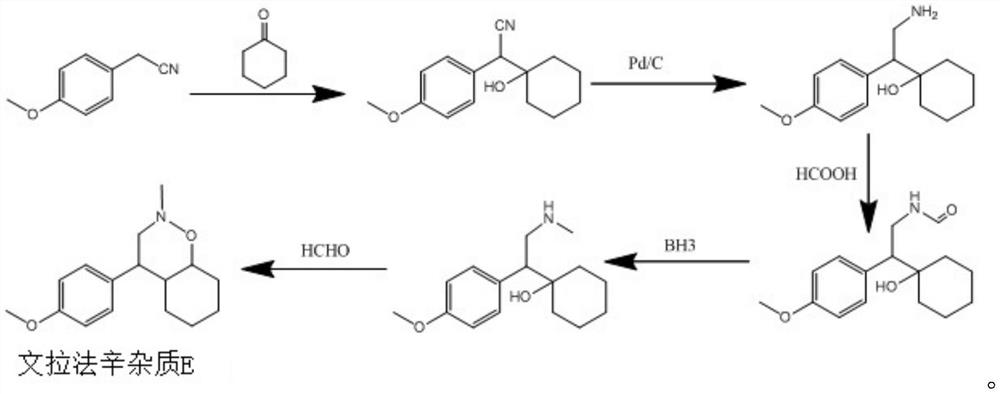
[0024] The method for preparing venlafaxine impurity E of the present invention includes:
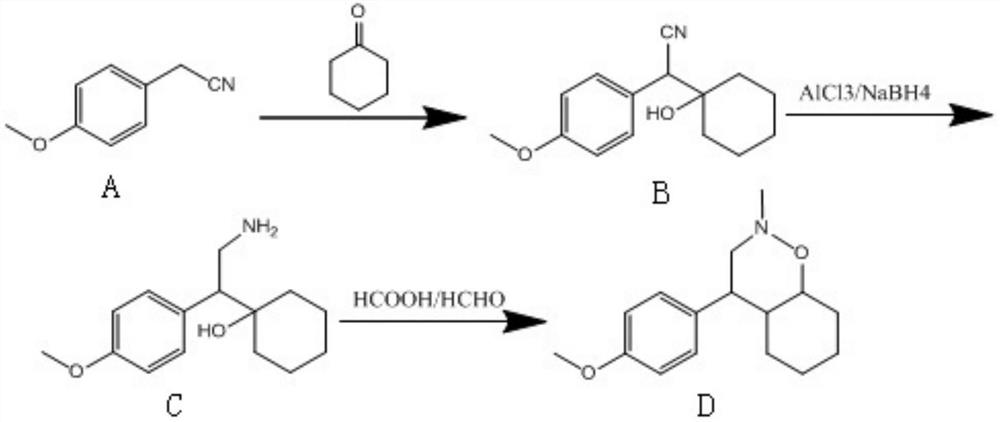
[0025] (1) Condensation reaction: Dissolve 4-methoxybenzeneacetonitrile (A) in ethanol, strengthen alkali to make the solution alkaline, add cyclohexanone, react at 15-30℃ for 4~5h, then use acid Adjust the pH to 5-7 and quench, and the product is filtered and dried to obtain 1-[2-cyano-1-(4-methoxyphenyl)ethyl]cyclohexanol (B);
[0026] (2) Reduction reaction: add aluminum trichloride and sodium borohydride to the solvent tetrahydrofuran, stir for 1h, add 1-[2-cyano-1-(4-methoxyphenyl)ethyl obtained in step (1) Cyclohexanol (B), reflux reaction for 3~4h, add acid to quench, extract with ethyl acetate, and distill the oil phase to obtain 1-[2-amino-1-(4-methoxyphenyl)ethyl ]Cyclohexanol (C);
[0027] (3) Ring-forming reaction: add formic acid and formaldehyde to the 1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol (C) obtained in step (2), at 50~ After reacting at 80°C for 2 to 3 hours, the...
Embodiment 1~5
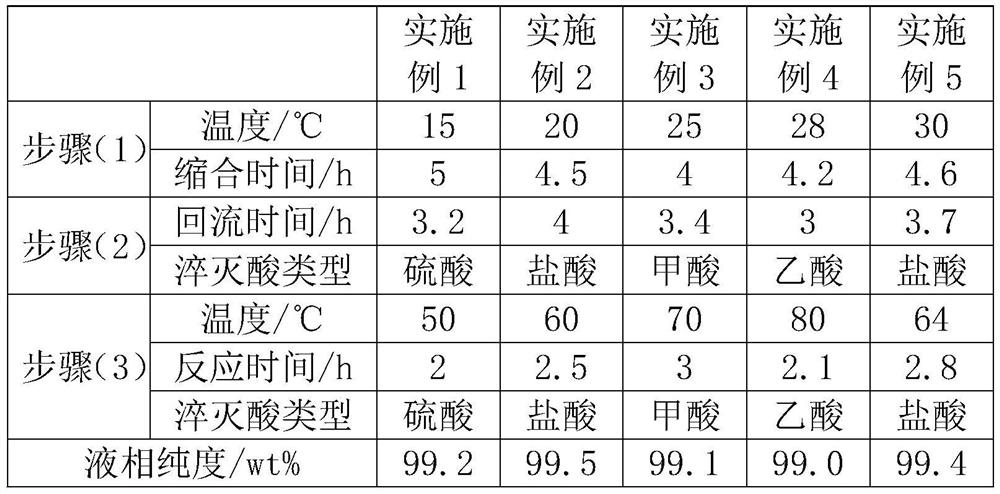
[0031] The specific reaction conditions and liquid phase purity of Examples 1 to 5 are as follows:
[0032]
[0033] It can be seen from the above table that the liquid phase purity of the present invention is greater than or equal to 99%, the method for synthesizing venlafaxine impurity E has fewer steps and higher product purity. The method avoids the use of high-pressure hydrogenation equipment and dangerous chemical reagents, and has high safety. In addition, there is less waste liquid, simple post-reaction treatment, convenient operation, and convenient industrial production.
[0034] Step (1) The strong base is sodium hydroxide, and the amount ratio to 4-methoxybenzeneacetonitrile is 1:1. Step (1) The ratio of cyclohexanone to 4-methoxybenzeneacetonitrile is 1 to 3:1. In the step (2), the amount ratio of aluminum trichloride to 1-[2-cyano-1-(4-methoxyphenyl)ethyl]cyclohexanol is 2.2-5.5:1, boron The ratio of sodium hydride to 1-[2-cyano-1-(4-methoxyphenyl)ethyl]cyclohexano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com