Medicine for preventing or treating ophthalmic disease associated with enhanced intraocular neovascularization and/or intraocular vascular permeability
A technology of vascular permeability and angiogenesis, which can be applied to cardiovascular system diseases, sensory diseases, drug combinations, etc., and can solve problems such as ophthalmic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
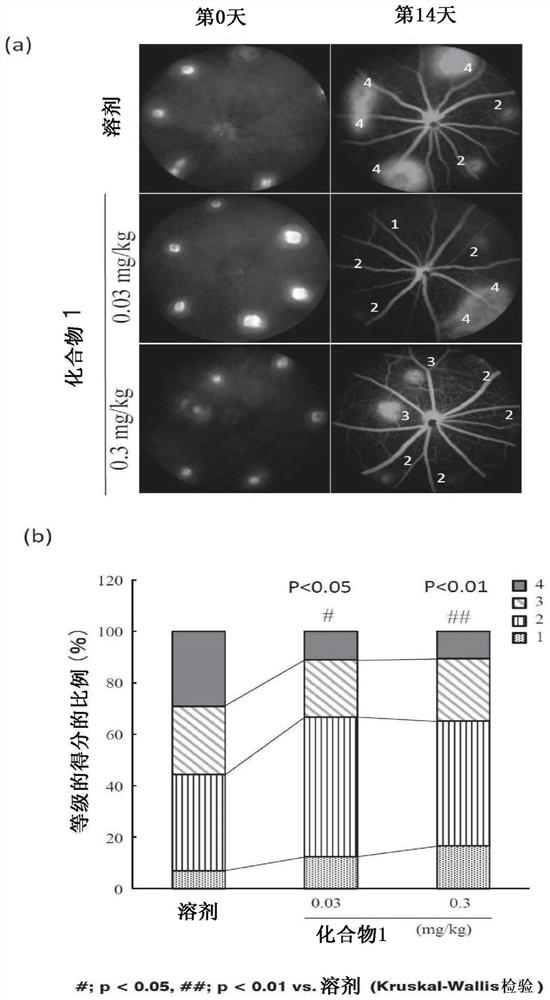
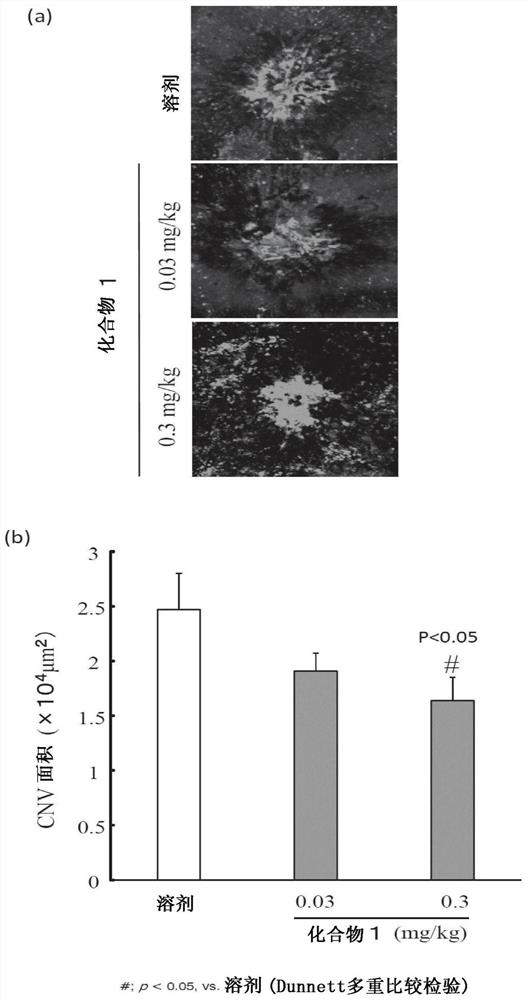
[0154] (Example 1) Effect on laser-induced choroidal neovascularization (choroidal neovascularization, CNV) model
[0155] (1) Evaluation of fundus fluorescein angiography (FA) grade
[0156] The right eye of an 8-week-old mouse (Nippon SLC, C56BL / 6J strain) was instilled with Mydrin P eye drops (Santen Pharmaceutical, Mydrin is a registered trademark) to dilate the pupil. The 7:1 mixed anesthetic solution of ketamine and xylazine was diluted 10 times with normal saline, and 10 mL / kg of the resulting solution was administered to the thigh muscle. Then, eye drops were instilled with Hyalein (registered trademark) eye drops 0.1% (Santen Pharmaceutical Co., Ltd.) so as not to dry the eyeballs.
[0157] Then, put the cover glass close to the right eye to observe the fundus, and use a laser photocoagulation device (MC500; NIDEKCO.LTD, Aichi, Japan) to irradiate 6 parts of the laser at equal intervals on the circumference of the optic nerve head (wavelength : 647nm, spot size: 50μ...
Embodiment 2
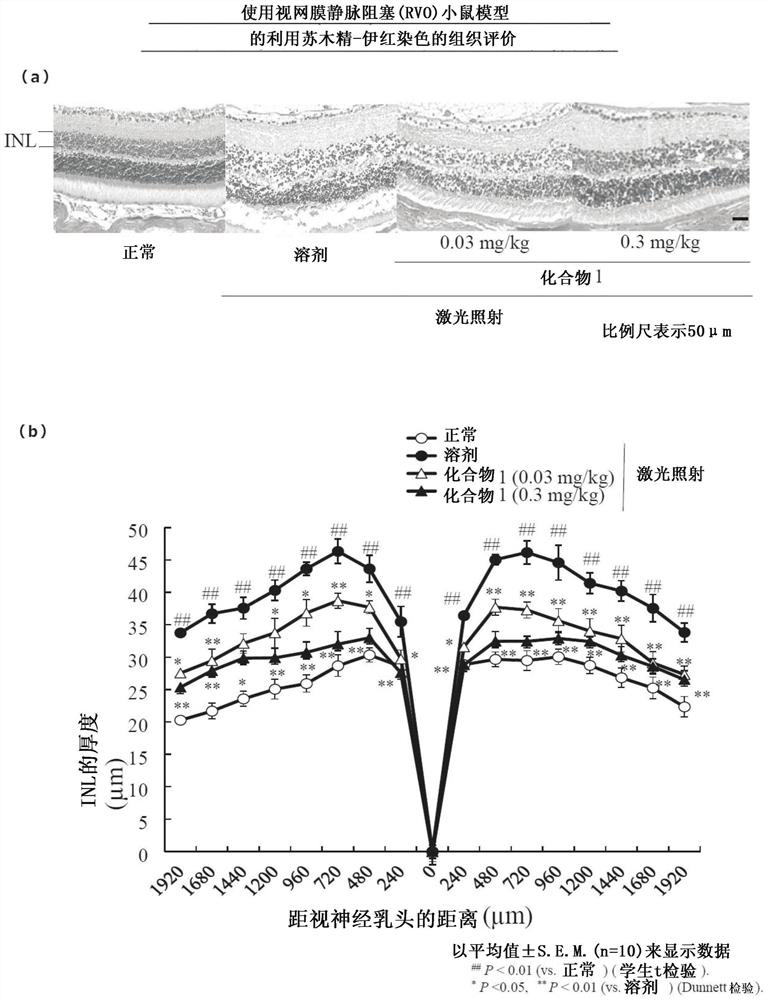
[0170] (Example 2) to the effect of retinal vein occlusion (RVO) model
[0171] Eight-week-old mice (Nippon SLC, ddy series) were anesthetized by intramuscularly administering 10 mL / kg of a mixed anesthetic solution diluted to ketamine (120 mg / kg) and xylazine (6 mg / kg). Then, 0.15 mL of rose bengal (20 mg / kg; WAKO) was administered into the tail vein, and the retinal vein was occluded by irradiating laser light to the vein three papillary from the optic nerve head in the right eye of the mouse. It should be noted that, when blocked, 10 to 15 laser irradiations are performed on one vein. For laser irradiation, an accessory (wavelength: 532 nm, spot size: 50 μm, irradiation time: 5000 ms, laser output power: 50 mW) of the fundus imaging device Micron4 (Phoenix Research Laboratories, Inc., Preston, CA, USA) was used. Three veins were occluded in each eye, and complete occlusion of the veins after laser irradiation was confirmed by fundus photographs.
[0172] Compound 1 was su...
reference example 1
[0175] (Reference Example 1) Correlation with Lymphopenia
[0176] For 7-week-old mice (Japan SLC Company, C57BL / 6J series), compound 1 suspended in a solvent (0.5% MC solution) was used at 0.003, 0.01, 0.03, 0.1, 0.3 or 1 mg / kg (free body conversion) The same dosage as in Example 1 was repeatedly administered orally once a day for 14 days. As a control, only solvent was administered (solvent group). Experiments were carried out using 5 cases respectively.
[0177] At 24 hours after the final administration, blood was collected from a large abdominal vein using a heparin-treated syringe under isoflurane anesthesia, and mixed with dipotassium ethylenediaminetetraacetic acid (EDTA·2K) (1 mg). The number of lymphocytes in the collected blood was measured using XT-2000iv (Sysmex). show the result in Figure 4 (a). Compound 1 significantly decreased lymphocytes in peripheral blood at 0.1 mg / kg or more, but no significant effect was confirmed at 0.03 mg / kg.
[0178] Next, for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com