Method for synthesizing 5-nitrosalicylaldehyde
A technology of nitrosalicylaldehyde and p-nitrophenol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of polyphosphoric acid viscosity, unfavorable tracking and detection reaction, and difficult 5-nitro Salicylaldehyde industrial production, complex reaction process and other problems, to overcome the difficulty of tracking analysis and detection of reaction process, improve the efficiency of synthesis reaction, the effect of simple post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
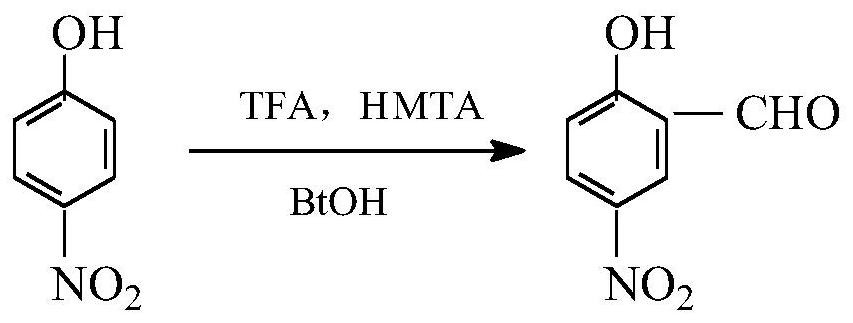
[0024] The present invention is a kind of method of synthesizing 5-nitro salicylaldehyde (reaction formula sees attached figure 1 ), is to take p-nitrophenol as a raw material, and is prepared by improving Duff reaction and hexamethylenetetramine reaction, comprising the following steps:
[0025] S1: Add hexamethylenetetramine (14g, 0.1mol), p-nitrophenol (14g, 0.1mol) and 13mL trifluoroacetic acid into a four-necked flask, stir, slowly raise the temperature, and add the remaining 17mL of trifluoroacetic acid (30mL in total, 0.4mol), heated to 70°C for reflux reaction for 6 hours to obtain a reaction product mixture;
[0026] S2: After concentrating the reaction product mixture in step S1 to remove part of the trifluoroacetic acid, adding sodium bicarbonate aqueous solution to adjust the pH of the reaction product mixture to about 6, cooling and standing for a period of time, a light yellow solid precipitates, and successively filter, Washing and drying gave 12.1 g of a light...
Embodiment 2
[0029] The present invention is a kind of method of synthesizing 5-nitro salicylaldehyde (reaction formula sees attached figure 1 ), is to take p-nitrophenol as a raw material, and is prepared by improving Duff reaction and hexamethylenetetramine reaction, comprising the following steps:
[0030] S1: Add hexamethylenetetramine (21g, 0.15mol), p-nitrophenol (14g, 0.1mol) and 15mL trifluoroacetic acid into a four-necked flask, stir, slowly raise the temperature, and add the remaining 22.5 mL of trifluoroacetic acid (37.5 mL in total, 0.5 mol) was heated to 78°C for reflux reaction for 7 hours to obtain a reaction product mixture;
[0031] S2: After concentrating the reaction product mixture in step S1 to remove part of the trifluoroacetic acid, adding sodium bicarbonate aqueous solution to adjust the pH of the reaction product mixture to about 6, cooling and standing for a period of time, a light yellow solid precipitates, and successively filter, After washing and drying, 10.6...
Embodiment 3
[0033] The present invention is a kind of method of synthesizing 5-nitro salicylaldehyde (reaction formula sees attached figure 1 ), is to take p-nitrophenol as a raw material, and is prepared by improving Duff reaction and hexamethylenetetramine reaction, comprising the following steps:
[0034] S1: Add hexamethylenetetramine (28g, 0.2mol), p-nitrophenol (14g, 0.1mol) and 13mL trifluoroacetic acid into a four-necked flask, stir, slowly raise the temperature, and add the remaining 17mL of trifluoroacetic acid (30mL in total, 0.4mol), heated to 70°C for reflux reaction for 7 hours to obtain a reaction product mixture;
[0035] S2: After concentrating the reaction product mixture in step S1 to remove part of the trifluoroacetic acid, adding sodium bicarbonate aqueous solution to adjust the pH of the reaction product mixture to about 6, cooling and standing for a period of time, a light yellow solid precipitates, and successively filter, After washing and drying, 11.3 g of a lig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com