Diclofenac enteric-coated tablet and processing technology thereof
A technology of diclofenac and processing technology, applied in the field of diclofenac enteric-coated tablet and its processing technology, can solve problems such as affecting the qualified rate, and achieve the effect of improving the qualified rate of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
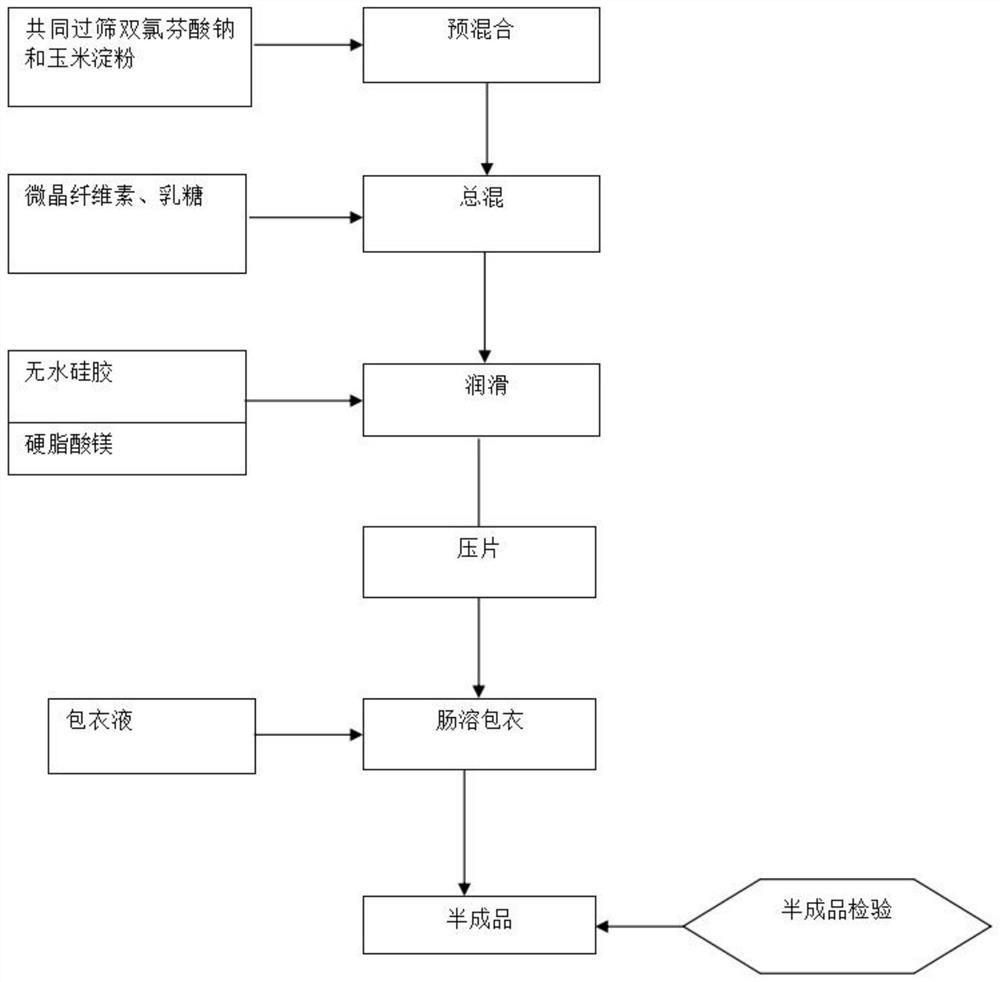
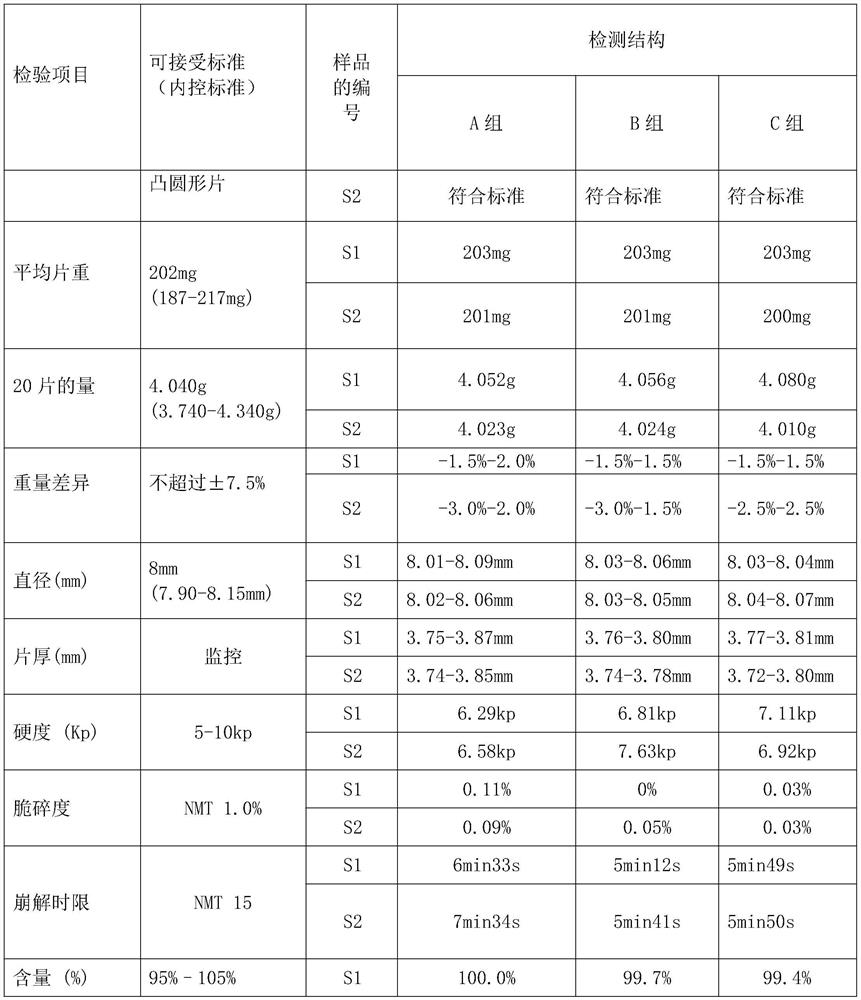
[0023] A diclofenac enteric-coated tablet, comprising the following raw materials in parts by weight: 25 parts of diclofenac sodium, 48 parts of lactose, 35 parts of cornstarch, 38 parts of microcrystalline cellulose, 2 parts of anhydrous silica gel, 2 parts of magnesium stearate, and cellulose vinegar 11.64 parts of French esters, 0.466 parts of polyethylene glycol, 1.242 parts of castor oil, 1.653 parts of Opadry, 2.3 parts of acetone, and 2.3 parts of dichloromethane.
[0024] A kind of processing technology of diclofenac enteric-coated tablet, following processing steps:
[0025] S1. Weigh the raw materials according to the proportion;
[0026] S2. Diclofenac sodium and cornstarch that have passed through a 30-mesh sieve are added to the mixer and mixed for 10 minutes;
[0027] S3. Add microcrystalline cellulose and lactose that have passed through a 30-mesh sieve into the mixer, and continue mixing for 10 minutes;
[0028] S4. Pass anhydrous silica gel through a 30-mesh...
Embodiment 2
[0031] A diclofenac enteric-coated tablet, comprising the following raw materials in parts by weight: 45 parts of diclofenac sodium, 54 parts of lactose, 37 parts of cornstarch, 39.6 parts of microcrystalline cellulose, 1.8 parts of anhydrous silica gel, 3.6 parts of magnesium stearate, cellulose vinegar 18.532 parts of French esters, 0.618 parts of polyethylene glycol, 1.853 parts of castor oil, 2.631 parts of Opadry, 2.8 parts of acetone, and 2.8 parts of dichloromethane.
[0032] A kind of processing technology of diclofenac enteric-coated tablet, following processing steps:
[0033] S1. Weigh the raw materials according to the proportion;
[0034] S2. Diclofenac sodium and cornstarch that have passed through a 30-mesh sieve are added to the mixer and mixed for 10 minutes;
[0035] S3. Add microcrystalline cellulose and lactose that have passed through a 30-mesh sieve into the mixer, and continue mixing for 10 minutes;
[0036] S4. Pass anhydrous silica gel through a 30-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com