Preparation method of high-purity sorafenib tosylate crystal form III
A technology of toluenesulfonic acid and fenib crystal form, which is applied in the field of preparation of high-purity sorafenib toluenesulfonate crystal form III, can solve the problems of high content of toxic impurities, easy residual products, difficult to remove, etc. The effect of impurity content, improving safety and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0050] Preparation 1: N-[4-chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl ] Preparation of urea (compound II):
[0051]
[0052] 10g of 4-chloro-3-trifluoromethylphenyl isocyanate was dissolved in 100ml of dichloromethane to obtain solution 1, and 9.9g of N-methyl-4-(4-amino)phenoxypyridine-2-carboxamide Dissolve in 99ml of dichloromethane to obtain solution 2. Control the temperature at about 0°C, add solution 2 dropwise to solution 1, after the dropwise addition, stir the reaction at room temperature for 70 hours, filter with suction, wash the filter cake with dichloromethane, and dry under reduced pressure to obtain 16.6 g of a light yellow solid, yield 87.5%.
[0053] 1 H-NMR (400MHz, DMSO-d 6 ,δppm): 2.77(d,3H), 7.16(m,3H), 7.37(d,1H), 7.62(m,4H), 8.11(d,1H), 8.49(d,1H), 8.77(dd, 1H), 8.99(s,1H), 9.21(s,1H).
[0054] MS(m / z):M+H + =465.20
preparation example 2
[0055] Preparation 2: N-[4-chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl ] Preparation of urea p-toluenesulfonate (compound I) crystal form I
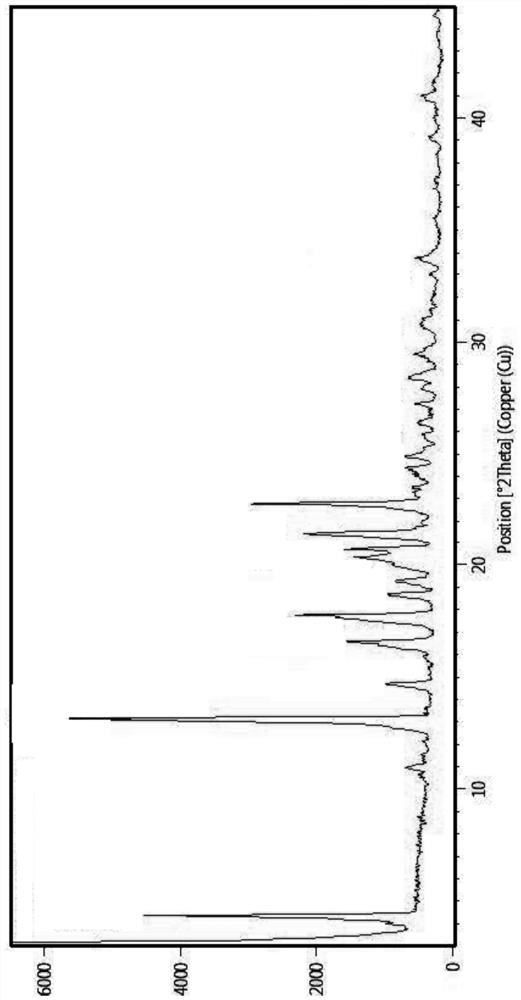
[0056] Add 2g of compound II into 20ml of acetonitrile, stir evenly, add 3.0ml of purified water and 1.2g of p-toluenesulfonic acid, heat to reflux to dissolve, cool down to 20-30°C, filter with suction, and dry in the air to obtain 2.4g of white solid , yield 87.6%. The product obtained is subjected to X-ray powder diffraction, and the result shows that the product is Compound I crystal form I, and the XRPD spectrum is shown in figure 1 shown.
Embodiment 1
[0057] Example 1: N-[4-chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl The preparation of urea p-toluenesulfonate (compound I) methanolate
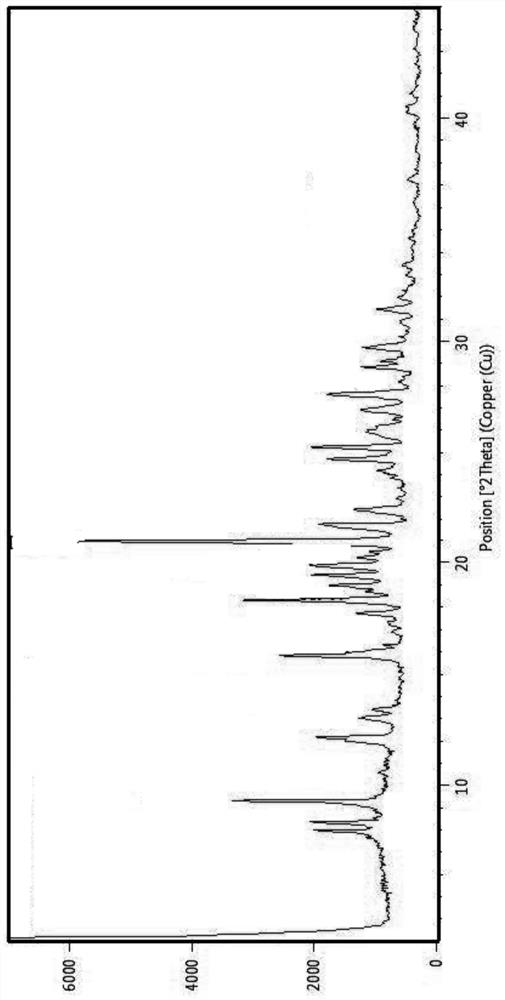
[0058] Add 20 g of compound I crystal form I to 100 ml of methanol, stir evenly, stir at 25 ° C for 2 h, filter with suction, and dry in the air to obtain 21 g of a white solid with a yield of 100%. X-ray powder diffraction is performed on the obtained product, and the results show that This product is the methanolate of compound I, XRPD pattern sees figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com