Method for co-production 2-methyl-6-nitrobenzoic acid and 3-nitro-2-methylbenzoic acid
A technology of toluic acid and nitrobenzoic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low selectivity and high risk of 3-nitro-o-xylene, To achieve the effect of easy recycling, high economic value and less waste salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 3-nitro-o-xylene (200 g) and 30% nitric acid (1000 g) into the oxidation reaction kettle, raise the temperature to 130-140°C, pass the oxygen pressure to 2.2-2.5 MPa, and keep stirring for 7 hours;
[0040] (2) Cool down to 20-30°C and filter to obtain 193 g of crude product (HPLC: 3-nitrophthalic acid 6.63%, 2-methyl-6-nitrobenzoic acid 15.92%, 3-nitro-2- Toluic acid 60.74%, 3-nitro-o-xylene 8.66%);
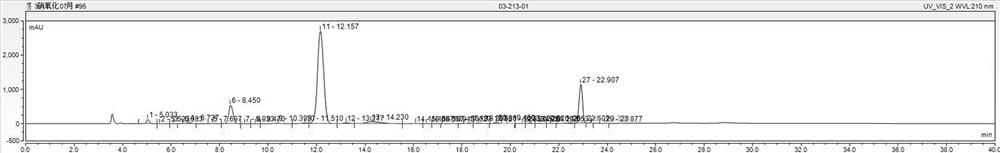
[0041] (3) The crude product was washed three times with 200 g of water to obtain 142 g of filter cake (HPLC spectrum as figure 1 Shown: 3-nitrophthalic acid 0.52%, 2-methyl-6-nitrobenzoic acid 12.3%, 3-nitro-2-methylbenzoic acid 72.03%, 3-nitro-o-xylene 7.1 %);
[0042] (5) Add 500 g of butanol and 5 g of p-toluenesulfonic acid to the crude product obtained in step 3, heat up and reflux for azeotropic dehydration, and start distillation to recover the solvent after the esterification reaction is complete.
[0043] (6) Cool the concentrated solution to 20-30°C,...
Embodiment 2
[0046] (1) Add 3-nitro-o-xylene (200 g) and 35% nitric acid (1000 g) into the oxidation reaction kettle, raise the temperature to 120-130 ° C, pass oxygen pressure 2.0-2.2 MPa, and keep stirring for 12 hours;
[0047] (2) Cool down to 10-20°C and filter to obtain 220 g of crude product (HPLC: 21.6% of 3-nitrophthalic acid, 24.7% of 2-methyl-6-nitrobenzoic acid, 3-nitro-2- Toluic acid 35.2%, 3-nitro-o-xylene 7.47%);
[0048] (3) The crude product was washed three times with 200 g of water to obtain a filter cake of 125 g (HPLC: 1.52% of 3-nitrophthalic acid, 24.2% of 2-methyl-6-nitrobenzoic acid, 3-nitro-2 -Toluic acid 52.03%, 3-nitro-o-xylene 7.05%);
[0049] (5) Add 800 g of methanol and 10 g of sulfuric acid to the crude product obtained in step 3, heat up to reflux for 4 hours, then continue to heat up to 80-90°C, and start distillation to recover methanol after the esterification reaction is complete.
[0050] (6) Heat the concentrated solution at 80-90°C, add 50 g of wa...
Embodiment 3
[0053] (1) Add 3-nitro-o-xylene (200 g) and 10% nitric acid (1200 g) into the oxidation reaction kettle, raise the temperature to 145-150 ° C, pass oxygen pressure 3.5-4.0 MPa, and keep stirring for 18 hours;
[0054] (2) Cool down to 75-85°C and filter while hot to obtain 90 grams of crude 3-nitro-2-methylbenzoic acid (HPLC: 2.71% 3-nitrophthalic acid, 2-methyl-6- Nitrobenzoic acid 6.76%, 3-nitro-2-methylbenzoic acid 80.04%, 3-nitro-o-xylene 5.36%), filtrate weight 1300 g, nitric acid concentration 5.5%;
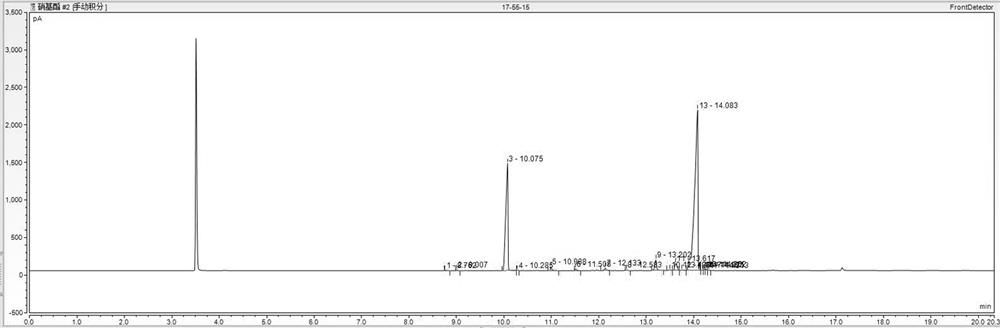
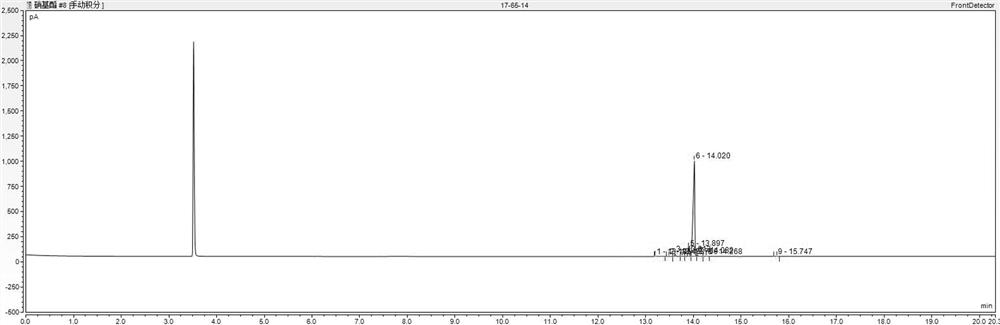
[0055] (3) The filter cake was washed with 80 g of water at 80°C, and the washing liquid was collected. Filter cake after water washing (HPLC spectrogram as Figure 4 As shown) weighed 75 g, added 75 g of anhydrous methanol, heated to 65 ° C to dissolve, filtered out the insoluble matter while it was hot, cooled the filtrate to crystallize, kept at 15-20 ° C for 1 hour, filtered and dried to obtain 3-nitro-2- 60 g of methyl benzoic acid finished product (HPLC spectrogram ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com