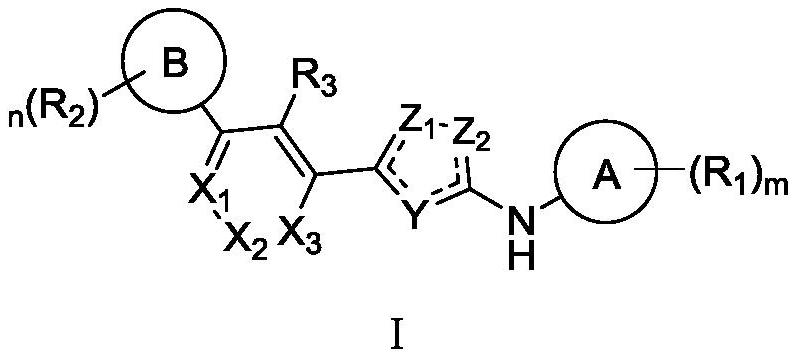
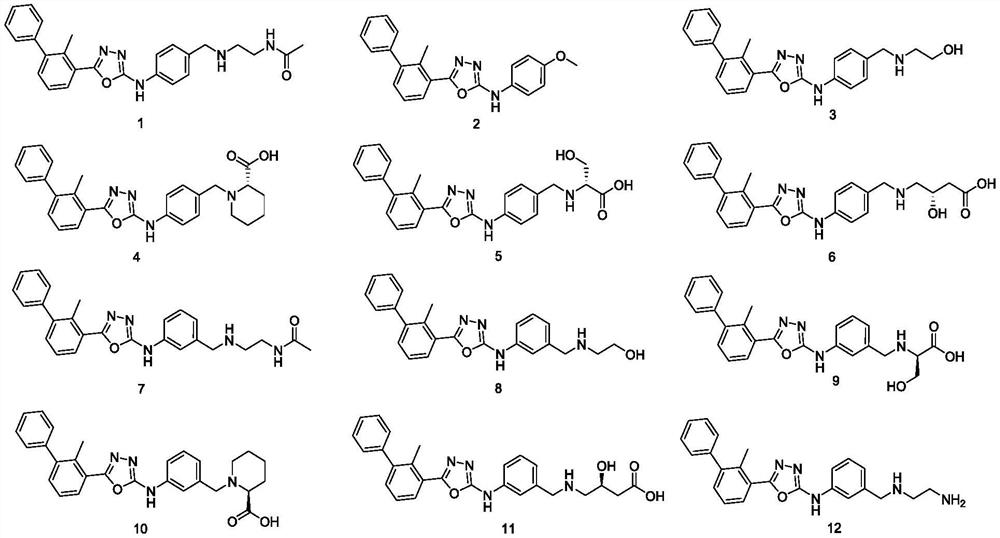
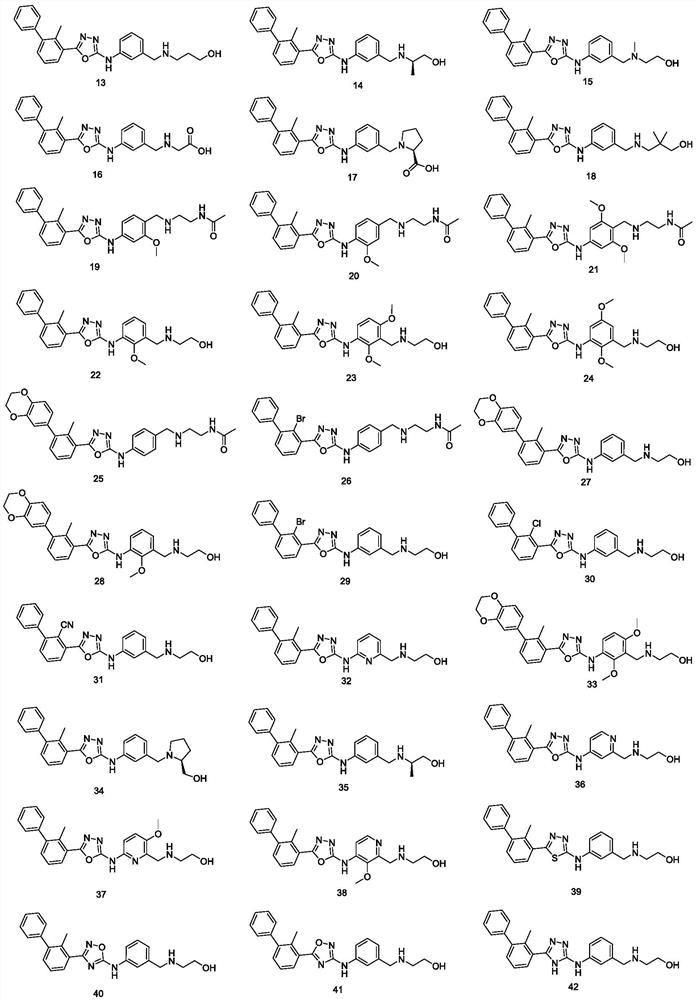Phenyl-substituted five-membered heterocyclic compounds as well as preparation method, application and pharmaceutical composition thereof
A five-membered heterocyclic, phenyl-substituted technology, applied in the fields of compounds and their preparation methods, uses and pharmaceutical compositions, can solve problems such as adverse reactions, expensive and difficult production, and difficult to control immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]
[0053] synthetic route:
[0054]
[0055] Synthesis of compound 1-A
[0056] Dissolve 2.58g of the compound 2-methyl-3-bromobenzoic acid in 20mL of 1:1 toluene / ethanol, then add 417mg of tetrakistriphenylphosphine palladium and 5mL of 2mol / L sodium carbonate aqueous solution, under nitrogen protection, stir at room temperature for 10min Then, 1.79 g of phenylboronic acid was added, and the mixture was moved to 80° C. and stirred overnight. TLC monitoring showed that the reaction of the raw materials was complete, and the reaction was stopped, the solvent was spin-dried, and 120 mL of ethyl acetate was added, washed once with water and once with saturated brine, 20 mL each time. The organic phase was concentrated and purified by column chromatography (the volume ratio of petroleum ether: ethyl acetate was 10:1) to obtain 2.2 g of compound 1-A.
[0057] Synthesis of compound 1-B
[0058] Dissolve 2 g of aminobenzyl alcohol and 5.38 g of triethylenediamine in 50...
Embodiment 2
[0068]
[0069] Referring to the synthesis method of Example 1, replacing p-aminobenzyl alcohol with p-methoxyaniline, Compound 2 can be prepared. 1 HNMR (300MHz, DMSO-d6) δ8.18(t, J=5.4Hz, 1H), 7.87(m, 2H), 7.65(d, J=7.6Hz, 2H), 7.59–7.35(m, 7H), 4.02(s,3H).
Embodiment 3
[0071]
[0072] Referring to the synthesis method of Example 1, replacing N-acetylethylenediamine with ethanolamine, Compound 3 can be prepared. 1 H NMR (300MHz, Methanol-d 4 )δ7.89–7.77(m,2H),7.59(dd,J=8.6,2.5Hz,3H),7.50–7.38(m,5H),7.37–7.26(m,2H),3.85(s,2H) ,3.72(t,J=5.6Hz,2H),2.80(t,J=5.2Hz,2H),2.51(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com