Artificial cardiac muscle tissue fibrosis model, and preparation method, preparation device and application thereof
A technique for tissue fibrosis and myocardium, applied in the field of biomedical engineering, can solve problems such as lack of myocardial infarction drugs in fibrosis models, research and development of human myocardial regeneration, and other problems, and achieve the effect of high-throughput drug testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
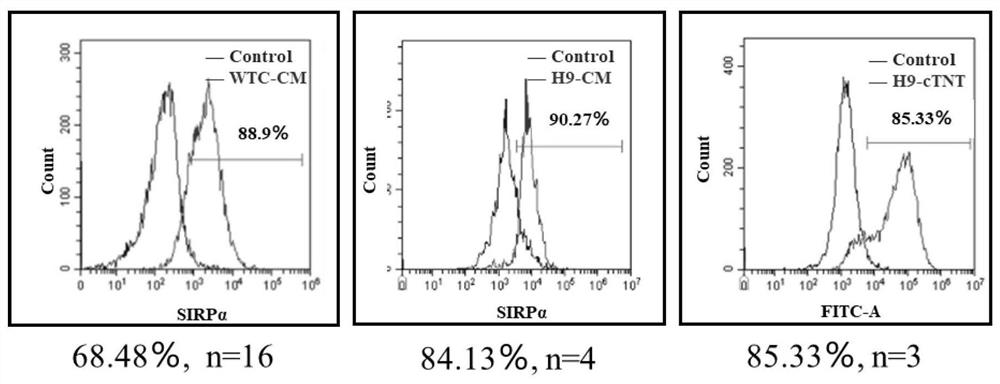
[0046] Example 1 Preparation of cardiomyocytes from pluripotent stem cells
[0047] The cells used in this example are human induced pluripotent stem cells or human embryonic stem cells, which are induced to differentiate into cardiomyocytes by using small molecules to regulate the WNT signaling pathway in stages, and the specific operations are as follows;
[0048] Human pluripotent stem cells were seeded into 6-well plates coated with Matrigel (Corning) at a seeding density of 3.0×10 6 cells / mL / well, using mTeSR medium (STEMCELL Technologies) in 5% CO 2 1. Cultivate in a constant temperature incubator (Thermo) at 37°C for 3 days until the cells grow to 70%-80% full. To prevent cell death, 10 μM Y27632 (Tocris) was added on the first day of culture.
[0049] Aspirate the old medium and replace it with cell culture medium I to initiate differentiation, in 5% CO 2 , and cultured in a 37°C constant temperature incubator (Thermo) for 2 days. Afterwards, discard the old medium...
Embodiment 2
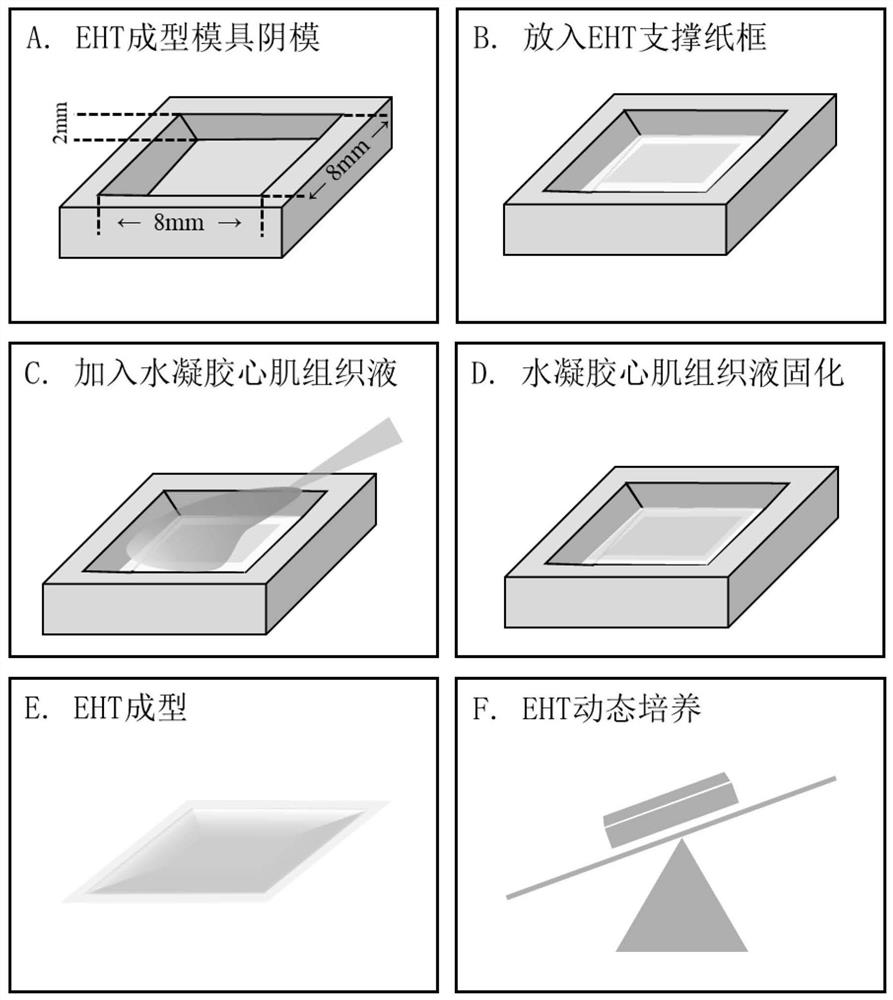
[0054] Example 2 Construction of engineered myocardial tissue sheet
[0055] Using natural extracellular matrix fibrinogen and Matrigel as the skeleton structure between cardiomyocytes, and then constructing engineered myocardial tissue, the specific operation is as follows:
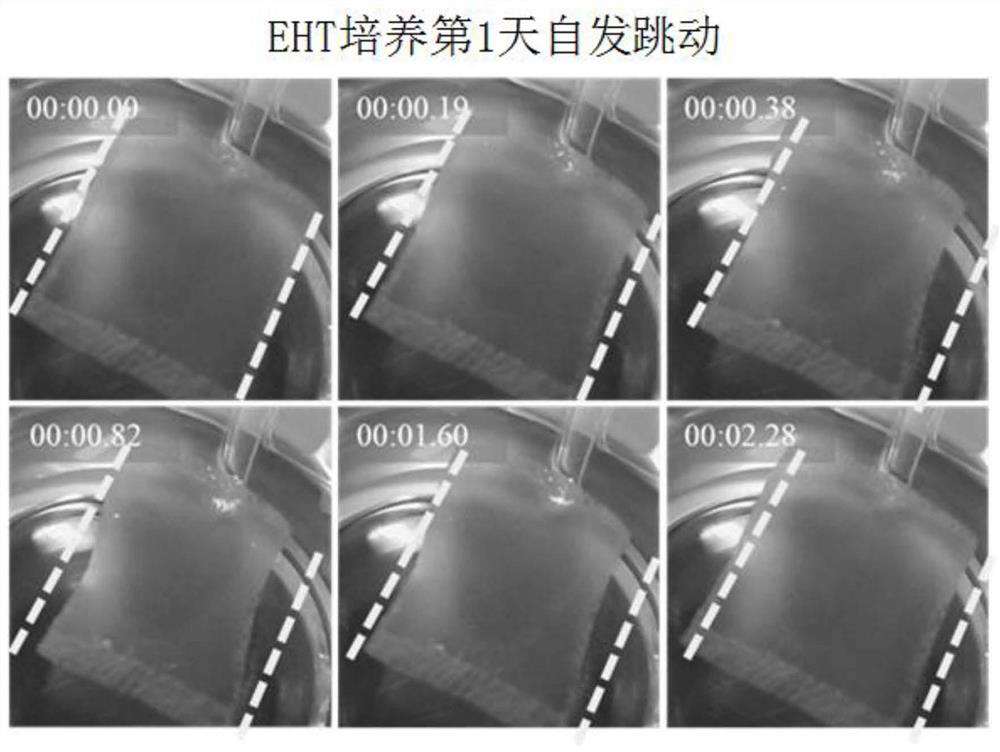
[0056] Use a fused deposition 3D printer (Aurora Corvo) to print the positive mold of the EHT molding mold with polylactic acid (PLA) as the material. Weigh 72 grams of PDMS base solution and add 8 grams of supporting cross-linking agent (SYLGARD TM 184 Silicone Elastomer Kit) was fully stirred and mixed, poured into a glass jar with the obtained male mold, and cured PDMS at 65°C for 4 hours. Separate the male mold, remove the excess cured PDMS, and leave the female mold of the EHT molding mold, such as figure 2 As shown in A. The negative mold was sterilized by high-pressure steam at 121°C for 20 minutes, and the PDMS negative mold was infiltrated with 1‰ F-127 for 30 minutes to increase the hydroph...
Embodiment 3
[0065] Example 3 Freezing injury and self-repair of engineered myocardial tissue slices
[0066] Use a 3D printer (Photon) to make a damage device with light-cured resin, such as Figure 4 A, specifically, a columnar injury device with a pointed end set on the base. Use a 3D printer to make a positive model of the damaged base with light-cured resin.
[0067] Weigh 72 grams of PDMS stock solution, add 8 grams of cross-linking agent, stir and mix well, pour it into a glass cylinder with a male mold of the damaged base, and cure PDMS at 65°C for 4 hours. Separate the male mold of the damaged base, remove the excess cured PDMS, and leave the female mold of the damaged base, such as Figure 4 b. Sterilize the female mold with the damaged bottom at 121°C for 20 minutes by high-pressure steam, wash it with sterile water three times, dry it completely, and set it aside for use.
[0068] Pour the PDMS into the wells of the 24-well plate, cover the bottom, and cure at 65 °C. The o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com