Binuclear vanadium catalyst as well as preparation method and application thereof
A nuclear vanadium catalyst and solvent technology, applied in the field of olefin polymerization organometallic catalyst and olefin coordination polymerization, can solve the problems of poor antistatic compatibility, limited application scope, low hygroscopicity, etc., and achieves easy reaction and rich application. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
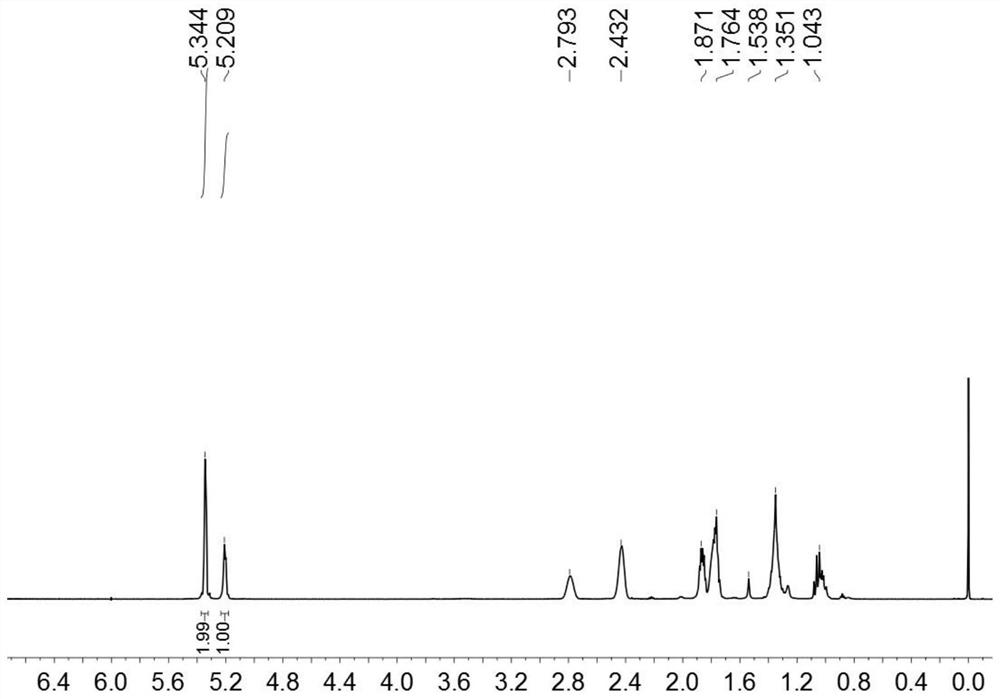
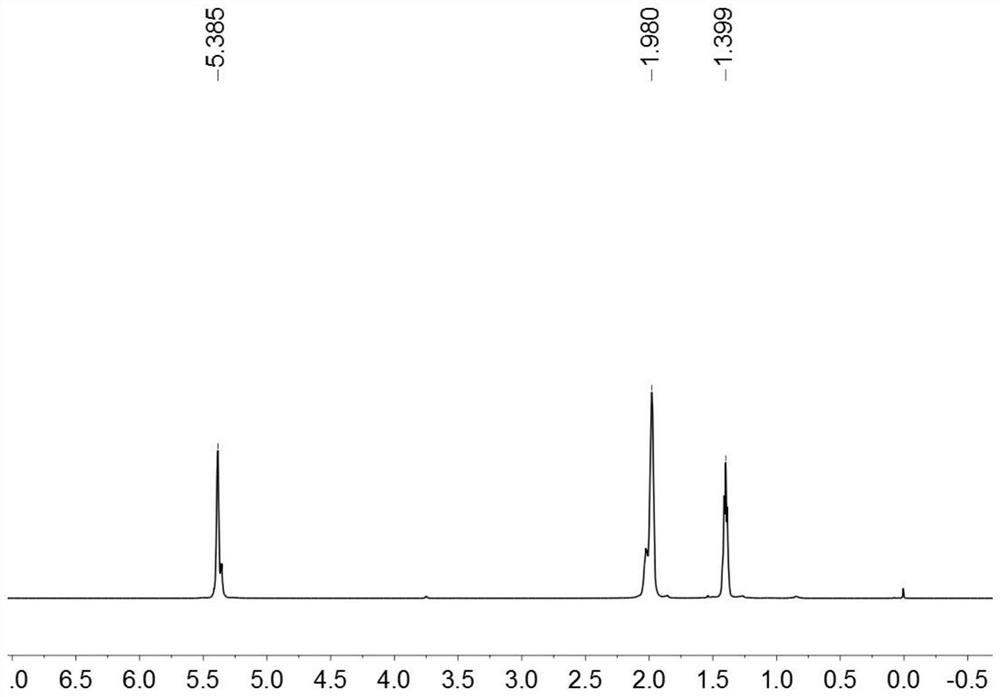
[0044] (1) Preparation of binuclear vanadium trichloride: under nitrogen protection, weigh 2,6-dimethylisocyanate (3.54g, 20mmol) in a Schlenk bottle, add solvent n-octane (15mL), slowly add trichloro For vanadyl, put the Schlenk bottle in an oil bath, stir at 120°C for 12h, filter through diatomaceous earth to obtain a dark green solution, remove the solvent by vacuum, wash with n-hexane and DCM, stand at -5°C for 6h, vacuum The solvent was removed to give dark green binuclear vanadium trichloride (3.76g, 40%);
[0045](2) Preparation of binuclear vanadium trialkylate: Weigh the product (150mg, 0.54mmol) of step (1) into a reaction flask, add solvent toluene (5mL), let stand at -5°C for 1h, slowly add alkyl Lithium reagent (1.91g, 1.62mmol), after stirring at room temperature for 6h, filtered through diatomaceous earth to obtain a reddish-brown solution, and the solvent was removed in vacuo to obtain a reddish-brown oily trialkylate (202mg, 86%);
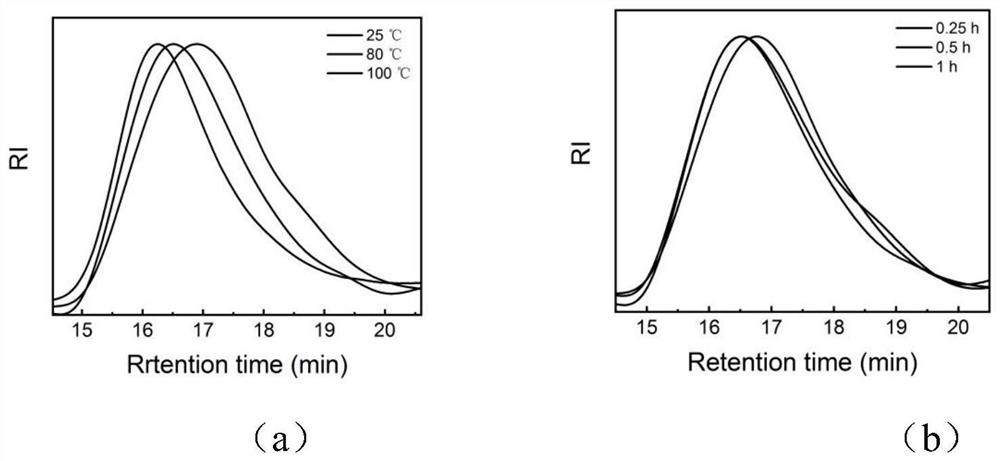
[0046] (3) Preparation of ...
Embodiment 2
[0051] (1) Preparation of binuclear vanadium trichloride: under nitrogen protection, weigh 2,6-dimethylisocyanate (3.54g, 20mmol) in a Schlenk bottle, add solvent n-octane (15mL), slowly add trichloro For vanadyl, put the Schlenk bottle in an oil bath, stir at 120°C for 12h, filter through diatomaceous earth to obtain a dark green solution, remove the solvent by vacuum, wash with n-hexane and DCM, stand at -5°C for 6h, vacuum The solvent was removed to give dark green binuclear vanadium trichloride (3.76g, 40%);
[0052] (2) Preparation of binuclear vanadium trialkylate: Weigh the product (150mg, 0.54mmol) of step (1) into a reaction flask, add solvent toluene (5mL), let stand at -5°C for 1h, slowly add alkyl Lithium reagent (1.91g, 1.62mmol), after stirring at room temperature for 6h, filtered through diatomaceous earth to obtain a reddish-brown solution, and the solvent was removed in vacuo to obtain a reddish-brown oily trialkylate (202mg, 86%);
[0053] (3) Preparation of...
Embodiment 3
[0058] (1) Preparation of binuclear vanadium trichloride: under nitrogen protection, weigh 2,6-dimethylisocyanate (3.54g, 20mmol) in a Schlenk bottle, add solvent n-octane (15mL), slowly add trichloro For vanadyl, put the Schlenk bottle in an oil bath, stir at 120°C for 12h, filter through diatomaceous earth to obtain a dark green solution, remove the solvent by vacuum, wash with n-hexane and DCM, stand at -5°C for 6h, vacuum The solvent was removed to give dark green binuclear vanadium trichloride (3.76g, 40%);
[0059] (2) Preparation of binuclear vanadium trialkylate: Weigh the product (150mg, 0.54mmol) of step (1) into a reaction flask, add solvent toluene (5mL), let stand at -5°C for 1h, slowly add alkyl Lithium reagent (1.91g, 1.62mmol), after stirring at room temperature for 6h, filtered through diatomaceous earth to obtain a reddish-brown solution, and the solvent was removed in vacuo to obtain a reddish-brown oily trialkylate (202mg, 86%);
[0060] (3) Preparation of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com