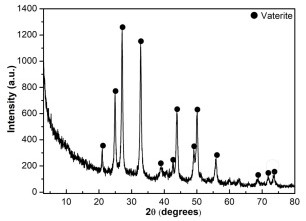
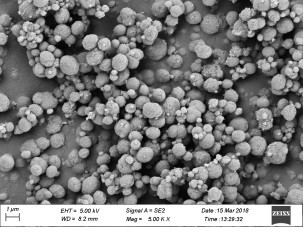
Preparation method of high-purity vaterite type calcium carbonate microspheres
A technology of vaterite and calcium carbonate, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of adding crystal form control agent, complicated preparation process and high cost of raw materials, and achieves low dosage, low preparation cost and low equipment investment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A preparation method of high-purity vaterite type calcium carbonate microspheres of the present invention comprises the following steps.
[0022] 1) Grinding the calcium sulfate raw material to obtain calcium sulfate powder with an average particle size of 60-180 mesh.
[0023] Wherein, the calcium sulfate raw material described in the above step 1) is mainly natural gypsum, gypsum tailings and industrial by-product gypsum, etc.
[0024] 2) Mix the calcium sulfate powder obtained in step 1) with the leaching aid, place in a stirred reaction vessel, and react for 30-120 minutes under normal pressure and 30-120 °C to obtain the reaction product.
[0025] Wherein, the leaching aid in step 2) is one or more of ammonium acetate, ammonium chloride and ammonium sulfate; the concentration of the leaching aid is 2-12 mol / L; the amount of the leaching aid is 5-20 mL / g.
[0026] 3) Suction filter, wash, and dry the reaction product obtained in step 2) to obtain a filtrate rich i...
example 1
[0033] The calcium sulfate raw material in the present embodiment selects phosphogypsum, and the main chemical composition of phosphogypsum is, by weight percentage: SO 3 38%, CaO32%, SiO 2 7%, other 23%; the specific process is as follows:
[0034] 1) Take 50g of the above-mentioned phosphogypsum powder, prepare 650 mL of 9.5 mol / L ammonium chloride solution, then pour the phosphogypsum powder into the ammonium chloride solution, stir and react at 90°C for 110 min, and suction filter after the reaction is completed Obtain filtrate and solid reactant;
[0035] 2) Take 500 mL of the above filtrate, add 29.4 g of calcium chloride powder and 45 mL of ammonia water to the solution, and pass through CO 2 gas, stirred at 85 °C for 95 min, CO 2 The flow rate is 210 mL / min, and the stirring speed is 400 r / min; after the reaction is completed, stop feeding CO 2 gas, the reaction product is suction filtered, washed and dried to obtain the calcium carbonate microsphere product;
[0...
example 2
[0038] Calcium sulfate raw material in the present embodiment selects gypsum tailings for use, and the main chemical composition of gypsum tailings is, by weight percentage: SO 3 24%, CaO20%, SiO 2 23%, Al 2 o 3 6%, Fe 2 o 3 3%, other 24%; the specific process is as follows:
[0039] 1) Take 20 g of the above gypsum tailings powder, prepare 240 mL of 4.5 mol / L ammonium acetate solution, then pour the gypsum tailings powder into the ammonium acetate solution, stir and react at 75 °C for 55 min, and suction filter after the reaction is completed Obtain filtrate and solid reactant;
[0040] 2) Take 200 mL of the above filtrate, add 3.6 g of calcium chloride powder and 18 mL of ammonia water to the solution, and pass in CO 2 gas, stirred the reaction at 55 °C for 70 min, CO 2 The flow rate was 85 mL / min, and the stirring speed was 250 r / min; after the reaction was completed, stop feeding CO 2 gas, the reaction product is suction filtered, washed and dried to obtain the cal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com