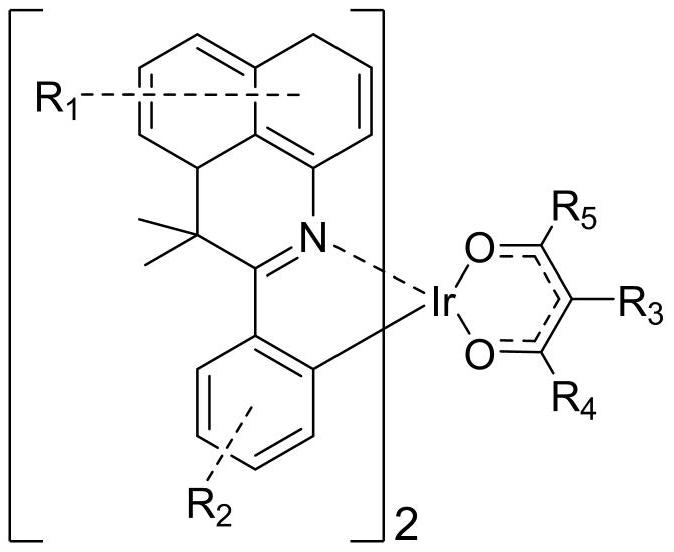
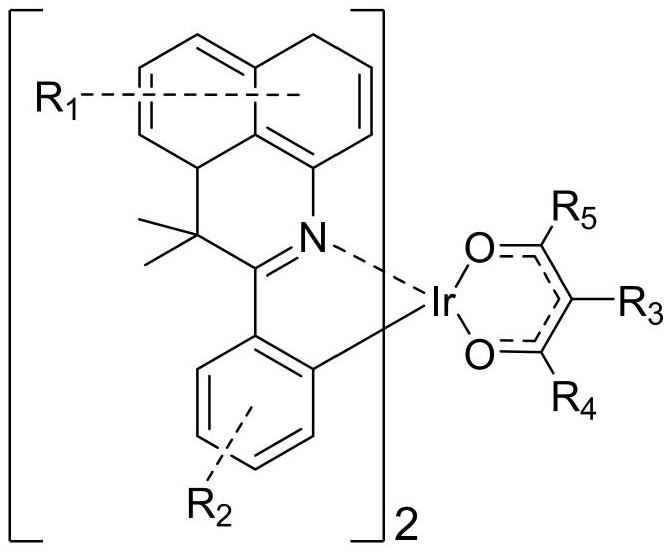
Organic iridium metal complex as well as preparation method and application thereof
An iridium metal complex, organic technology, applied in indium organic compounds, platinum group organic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of Compound F001
[0079]
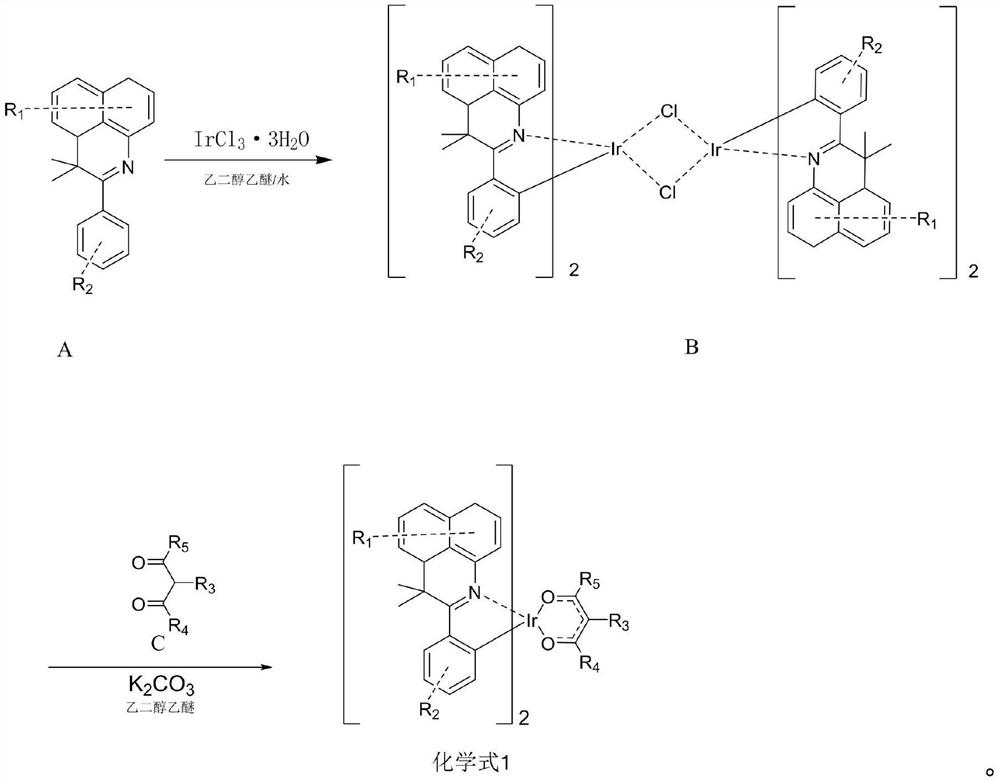
[0080] (1) Under nitrogen protection system, weigh A-001 (66.35mmol, 20g), IrCl 3 ·3H 2 O (22.12mmol, 7.8g) was added to the reaction system, a mixed solution of 600mL ethylene glycol ether and 200mL purified water was added, refluxed for 24 hours under the protection of nitrogen, and then cooled to room temperature, a precipitate was precipitated, and the precipitate was filtered with water, The bridging ligand B-001 (11.2 g, yield 61.12%) was obtained as a red powder by washing and drying with absolute ethanol and petroleum ether in sequence.
[0081] (2) Under the nitrogen protection system, weigh B-001 (6.76mmol, 11.2g), C-001 (20.28mmol, 2.03g), and then add 250mL of ethylene glycol ether and potassium carbonate (67.59mmol, 9.34g), under the protection of nitrogen, stirred at 120°C for 24 hours, suction filtered, washed with alcohol, dried, and used dichloromethane as solvent, followed by silica gel column chromatography...
Embodiment 2
[0088] Preparation of Compound F010
[0089]
[0090] (1) Under nitrogen protection system, weigh A-010 (37.04mmol, 20g), IrCl 3 ·3H 2 O (12.35mmol, 4.35g) was added to the reaction system, a mixed solution of 600mL ethylene glycol ether and 200mL purified water was added, refluxed for 24 hours under the protection of nitrogen, and then cooled to room temperature, a precipitate was precipitated, and the precipitate was filtered with water, The bridging ligand B-010 (10.4 g, yield 64.52%) was obtained as a red powder by washing and drying with absolute ethanol and petroleum ether in sequence.
[0091] (2) Under nitrogen protection system, weigh B-010 (3.98mmol, 10.4g), add C-010 (11.95mmol, 1.2g), then add ethylene glycol ethyl ether 250mL and potassium carbonate (39.83mmol , 5.51g), under the protection of nitrogen, stirred at 120°C for 24 hours, suction filtered, washed with alcohol, dried, and used dichloromethane as a solvent, followed by silica gel column chromatograp...
Embodiment 3
[0096] Preparation of Compound F012
[0097]
[0098] (1) Under nitrogen protection system, weigh A-012 (56.42mmol, 20g), IrCl 3 ·3H 2 O (18.81mmol, 6.63g) was put into the reaction system, a mixed solution of 600mL ethylene glycol ether and 200mL pure water was added, refluxed for 24 hours under the protection of nitrogen, and then cooled to room temperature, a precipitate was precipitated, the precipitate was suction filtered, and water , absolute ethanol, and petroleum ether were washed and dried in turn to obtain a red powder of bridging ligand B-012 (5.72 g, yield 60.88%).
[0099] (2) Under nitrogen protection system, weigh B-012 (5.72mmol, 10.7g), add C-012 (17.17mmol, 1.721g), then add ethylene glycol ethyl ether 250mL and potassium carbonate (57.24mmol , 7.91g), under the protection of nitrogen, stirred at 120°C for 24 hours, suction filtered, washed with alcohol, dried, used dichloromethane as solvent, and used silica gel column chromatography, the filtrate was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com