Group 4 avian adenovirus sd-f strain, inactivated vaccine and preparation method
A poultry adenovirus, SD-F technology, applied in the field of bioengineering, can solve the problems that hinder the research process of poultry adenovirus vaccines, the tumorigenicity of cells, the risk of vaccine tumorigenesis, etc., and achieve no tumorigenic risk, simple operation, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
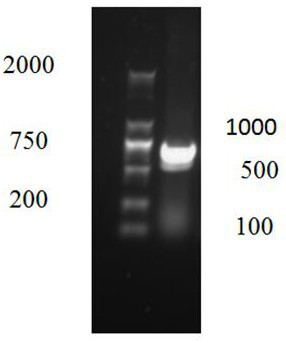
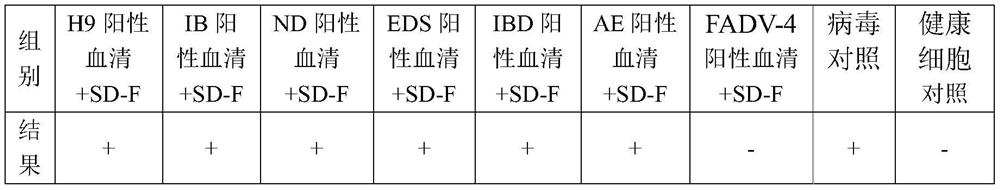
Image
Examples
Embodiment 1
[0021] This example describes the acquisition and specific identification of the SD-F strain of group I avian adenovirus type 4.
[0022] SPF eggs were purchased from Beijing Boehringer Ingelheim Viton Biotechnology Co., Ltd. and hatched to 10 days of age.
[0023] DF-1 cells were purchased from ATCC, USA.
[0024] MEM cell culture medium, DMEM medium, calf serum, trypsin were purchased from GIBCO Company.
[0025] Preparation of chicken embryo fibroblasts: CEFs were prepared according to the appendix of the 2000 edition of the Regulations for Veterinary Biological Products of the People's Republic of China.
[0026] 1. Obtainment of SD-F strain of group Ⅰ avian adenovirus type 4
[0027] Group Ⅰ avian adenovirus type 4 SD2015 strain, deposited in the China Center for Type Culture Collection, the deposit number is CCTCC NO: V201645.
[0028] After thawing the SD2015 strain of group I avian adenovirus type 4, centrifuge at 12,000 rpm for 10 minutes, and take the supernatant ...
Embodiment 2
[0065] Example 2 Preparation of avian adenovirus inactivated vaccine
[0066] The preparation method of avian adenovirus inactivated vaccine comprises the following steps:
[0067] (1) Culture of DF-1 cells
[0068] The formula of cell growth medium: add calf serum with a final concentration of 5% (volume percentage) and penicillin-streptomycin solution with a final concentration of 1% (volume percentage) to the MEM culture medium, and adjust the pH to After 7.0 to 7.2, use 0.22um filter paper to filter and pack and set aside. The penicillin-streptomycin solution is an aqueous solution containing 1000 U / L penicillin and 1000 U / L streptomycin.
[0069] The DF-1 seed cells were taken out from liquid nitrogen, thawed quickly in a 37°C water bath, and cultured in cell growth medium for 2 to 3 days for recovery. 7 ) were passaged 1:3.
[0070] (2) Virus inoculation: After the passaged DF-1 cells in step (1) grew into a dense monolayer, the cell surface was washed once with PBS,...
Embodiment 3
[0078] Example 3 Immune efficacy of inactivated vaccine against SD-F strain of avian adenovirus type 4 of group I
[0079] According to the method of Example 2, three batches of inactivated vaccines of group I type 4 avian adenovirus SD-F strain (20170210, 20170211, 20170212 batches of vaccines) were prepared. The virus content of each batch of vaccines is ≥ 10 4.4 / Feather portion.
[0080] For each batch of vaccine, 20 21-day-old SPF chickens were taken as the immunization group, and 0.3 ml of inactivated vaccine of group I group 4 avian adenovirus SD-F strain was subcutaneously injected into the neck of each chicken; the other 20 chickens were used as control and were not immunized. From 21 to 28 days after immunization, 10 animals in the immunized group and the control group were challenged, and the challenge dose was 10 7.0 TCID 50 (0.3ml) of liver tissue toxicity. Among them, the preparation method of liver tissue virus is as follows: Inject 49-day-old SPF chickens ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com