Hairpin probe group based on enzyme assisted cascade cycle amplification, preparation method and use
A technology of hairpin probes and cascading cycles, applied in biochemical equipment and methods, recombinant DNA technology, microbial measurement/inspection, etc., can solve problems such as complex operating procedures and expensive equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
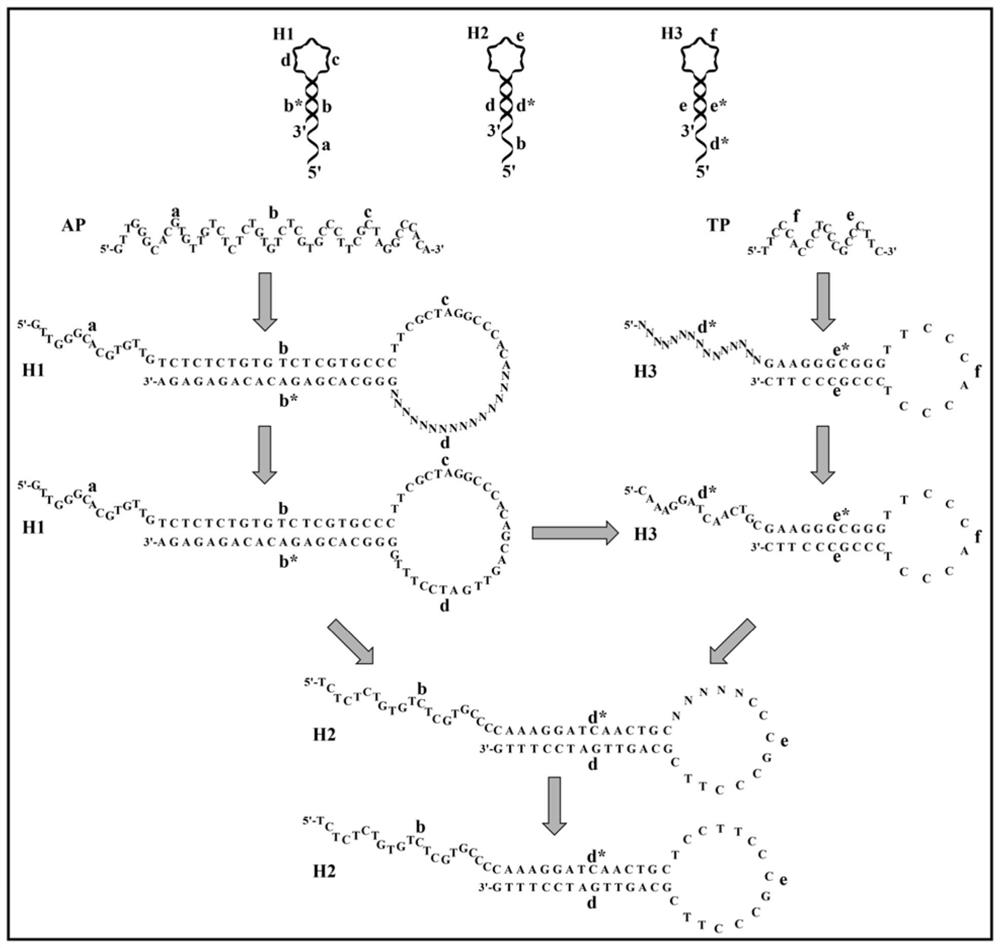
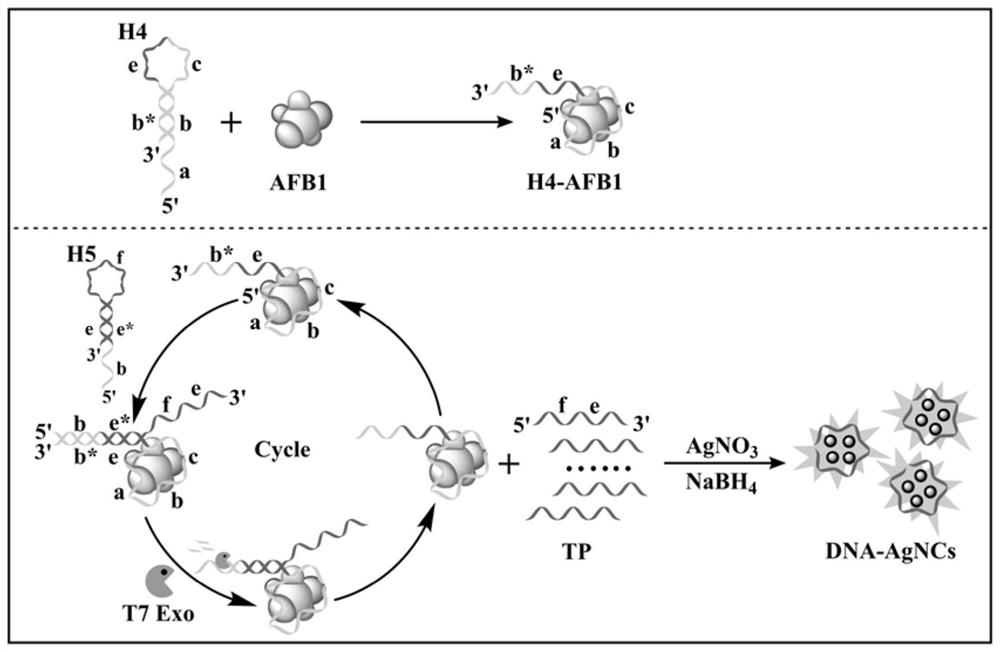
[0067] This embodiment provides a hairpin probe set based on enzyme-assisted cascade cyclic amplification, including the following probes:
[0068] The first hairpin probe, from the 5' end to the 3' end, sequentially includes an aptamer sequence that binds to a biological target and a first binding sequence, and part of the base pairing between the aptamer sequence and the first binding sequence makes the first hairpin probe Form a hairpin structure. Wherein, the aptamer sequence includes sequence a, sequence b and sequence c sequentially from the 5' end to the 3' end, and the first binding sequence includes sequence d sequentially from the 5' end to the 3' end, and sequence b complementary to sequence b * . Sequence b of the aptamer sequence and sequence b of the first binding sequence * The pairing forms the stem region of the hairpin probe, and the sequence d of the aptamer sequence and the sequence c of the first binding sequence form the stem loop region of the hairpin ...
Embodiment 2
[0079] This embodiment provides a method for preparing a hairpin probe set based on enzyme-assisted cascade cycle amplification, comprising the following steps:
[0080] Centrifuge the dry powder of hairpin probe sequences H1, H2 and H3 at 12000rpm for 5 minutes, and then dissolve them in 20mM Tris-HNO 3 Buffer (20mM NaNO 3 ,10mM NH4 NO 3 ,2mM Mg(NO 3 ) 2 , pH 7.4), a 100 μM stock solution was obtained. Next, stock solutions (100 μM) of hairpin probe sequences H1, H2 and H3 were heated to 95° C. for 5 minutes, and then slowly cooled to room temperature to form hairpin structures.
Embodiment 3
[0082] This embodiment provides a fluorescent detection method for a biological target, specifically aflatoxin B1 (AFB1), comprising the following steps:
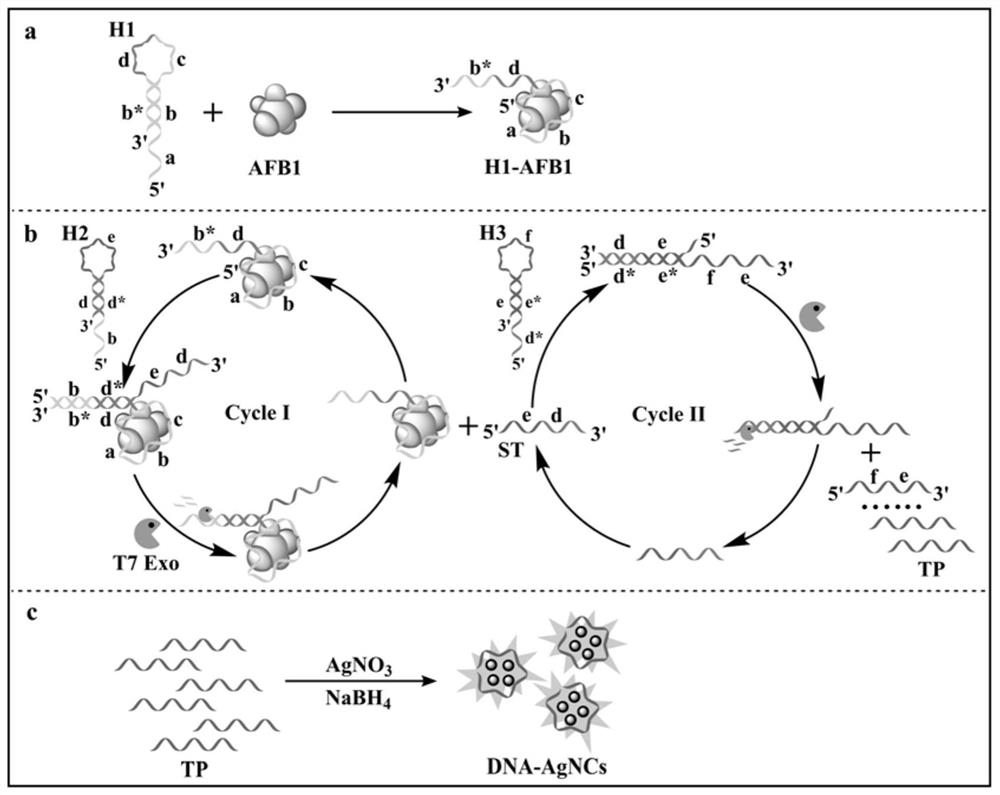
[0083] S1, mix the sample to be tested, the first hairpin probe, the second hairpin probe, the third hairpin probe and T7 exonuclease in the first buffer solution, heat the mixture and incubate, then cool to room temperature , to obtain the first reaction solution; specifically:
[0084] Mix 1 μL of the sample to be tested that may contain the target AFB1, 2 μL of the first hairpin probe H1 (1 μM), 2 μL of the second hairpin probe H2 (1 μM), 3 μL of the third hairpin probe H3 (1 μM) and 1 μL of T7 Exo (10U / μL) was sequentially added to 42μL of 1×NEBuffer 4 buffer (20mM Tris-HNO 3 ,50mM KNO 3 ,10mM Mg(NO 3 ) 2 , 1mM DTT, pH 7.9). The mixture was incubated at 37° C. for 90 minutes, and then cooled to room temperature to obtain the first reaction solution.
[0085] S2, taking the first reaction solution and adding it to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com