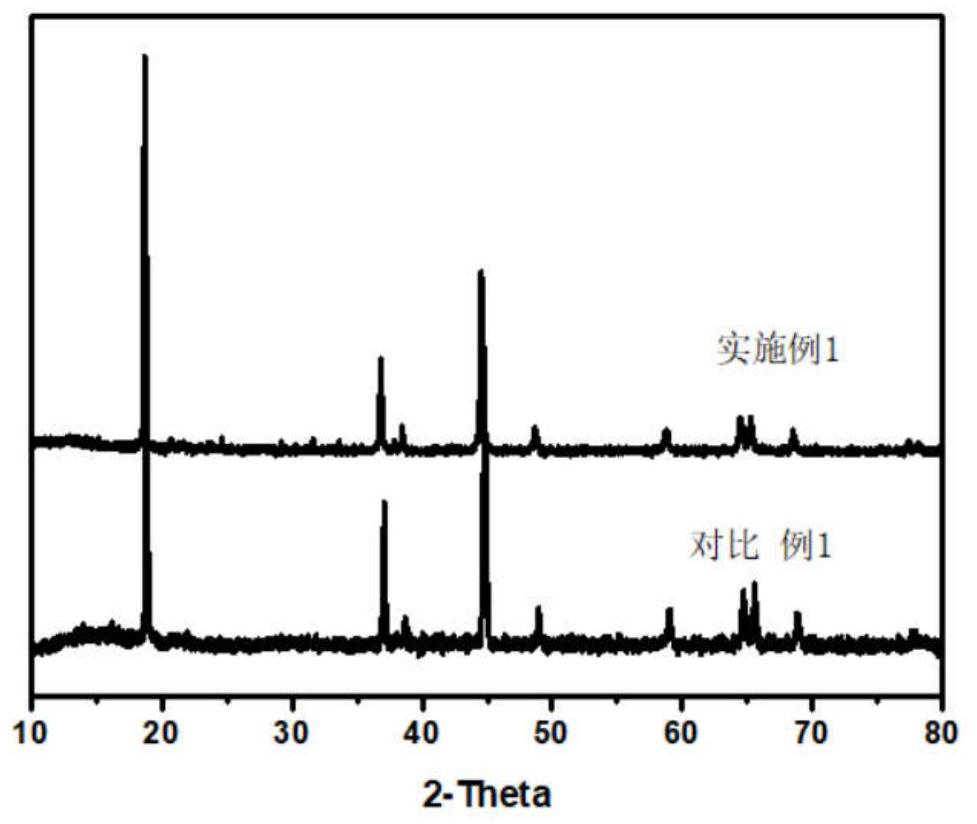
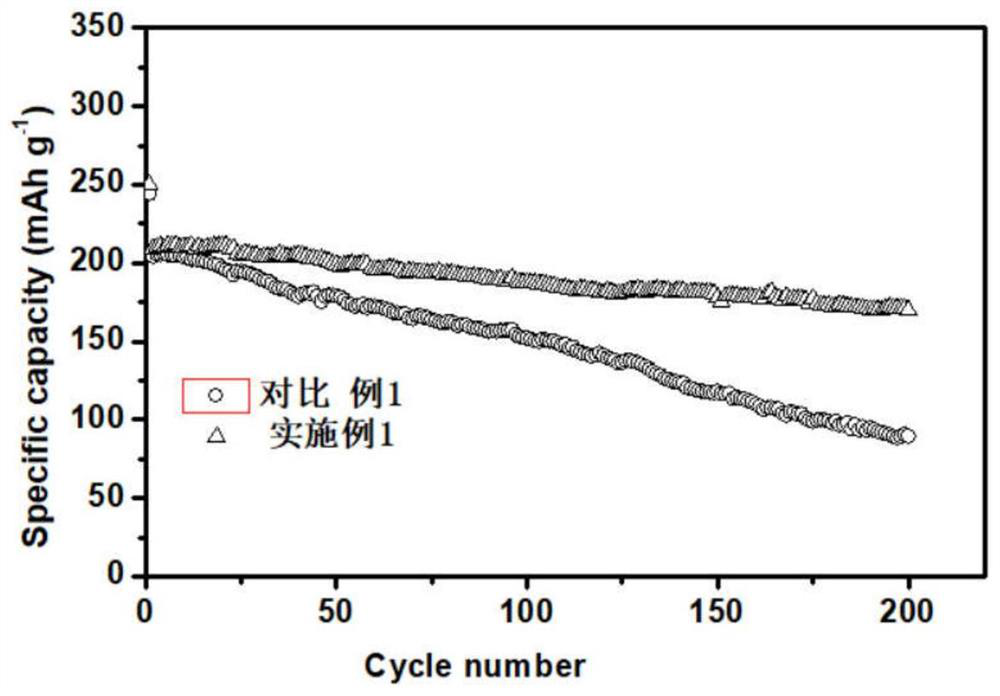
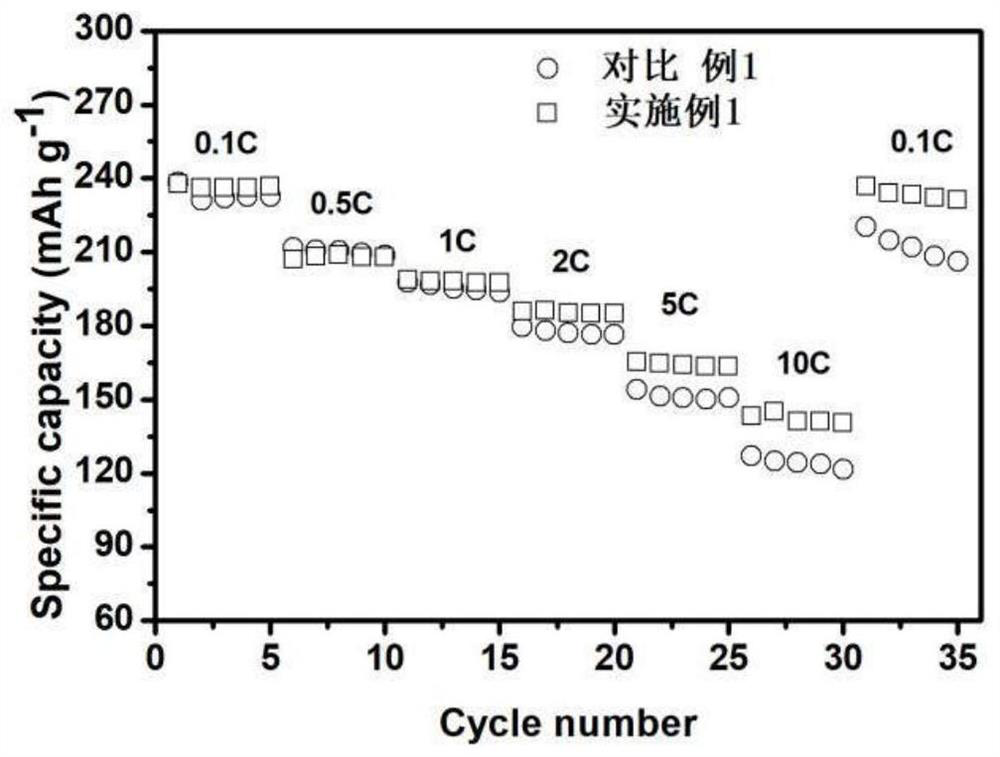
Ion conductor and heterostructure co-modified lithium ion battery positive electrode material and preparation method and application thereof
A lithium-ion battery, ion conductor technology, applied in battery electrodes, active material electrodes, positive electrodes, etc., can solve problems such as the lack of effective elimination of residual lithium on the surface of the material, the degradation of the internal structure of the material, and the limited effect of suppressing side reactions on the electrode surface.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Mix the lithium-rich material precursor with lithium carbonate, where TM:Li=1:1.5, mix evenly, sinter at 500 degrees for 5 hours, and then sinter at 900 degrees for 15 hours to obtain the lithium-rich manganese-based positive electrode material Li 1.2 mn 0.53 Ni 0.26 o 2 . 1g lithium-rich manganese-based cathode material Li 1.2 mn 0.53 Ni 0.27 o 2 Mix with 0.0224g diammonium hydrogen phosphate and 0.0495g cobalt nitrate in a solution of ethanol:water=3:1, Co:P:TM=1:1:50 (mass ratio), add acid:TM molar ratio is 1: 200 oxalic acid, mixed for 2 hours, evaporated the solution to dryness at 70°C, sintered the obtained solid powder in air, raised the temperature to 600°C at a rate of 2°C / min and kept it for 5h to obtain Li-Co-PO 4 Modified materials of cladding and spinel heterostructures. The modified lithium-rich manganese-based positive electrode material prepared by the above method, acetylene black and PVDF were uniformly mixed at a mass ratio of 8:1:1 to make a ...
Embodiment 2
[0051] Mix the lithium-rich material precursor with lithium carbonate, where TM:Li=1:1.5, mix evenly, sinter at 500 degrees for 5 hours, and then sinter at 900 degrees for 15 hours to obtain the lithium-rich manganese-based positive electrode material Li 1.2 mn 0.53 Ni 0.26 o 2 . 1g lithium-rich manganese-based cathode material Li 1.2 mn 0.53 Ni 0.27 o 2 Mix with 0.0112g diammonium hydrogen phosphate and 0.0365g aluminum nitrate in a solution of ethanol:water=3:1, Al:P:TM=1:1:100 (mass ratio), add acid: TM molar ratio is 1: 200 oxalic acid, mixed for 2 hours, evaporated the solution to dryness at 70°C, sintered the obtained solid powder in air, raised the temperature to 600°C at a rate of 2°C / min and kept it for 5h to obtain Li-Al-PO 4 Modified materials of cladding and spinel heterostructures. The retention rate of the modified material after 200 cycles is 83%, which is better than 44% of the original sample, and the rate performance is greatly improved. It can provid...
Embodiment 3
[0053] Mix the lithium-rich material precursor with lithium carbonate, where TM:Li=1:1.5, mix evenly, sinter at 500 degrees for 5 hours, and then sinter at 900 degrees for 15 hours to obtain the lithium-rich manganese-based positive electrode material Li 1.2 mn 0.53 Ni 0.26 o 2 . 1g lithium-rich manganese-based cathode material Li 1.2 mn 0.53 Ni 0.27 o 2 Mix with 0.0126g boric acid and 0.0365g manganese nitrate in a solution of ethanol:water=3:1, Mn:B:TM=1:1:100 (mass ratio), mix for 2 hours, and evaporate the solution to dryness at 70°C , the obtained solid powder was sintered in air, and the temperature was raised to 700°C at a rate of 2°C / min and kept for 5h to obtain Li-Mn-PO 4 Modified materials of cladding and spinel heterostructures. The retention rate of the modified material after 200 cycles is 78%, which is better than 44% of the original sample, and its capacity at high rate 10C is 142mAh / g, which is better than the original sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com