Pyrazine compounds with multiple effects and preparation method thereof
A compound, pyrazine technology, applied in the field of pyrazine compounds and their preparation, can solve problems such as lack of treatment methods, complex and diverse pathogenesis, hidden disease process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
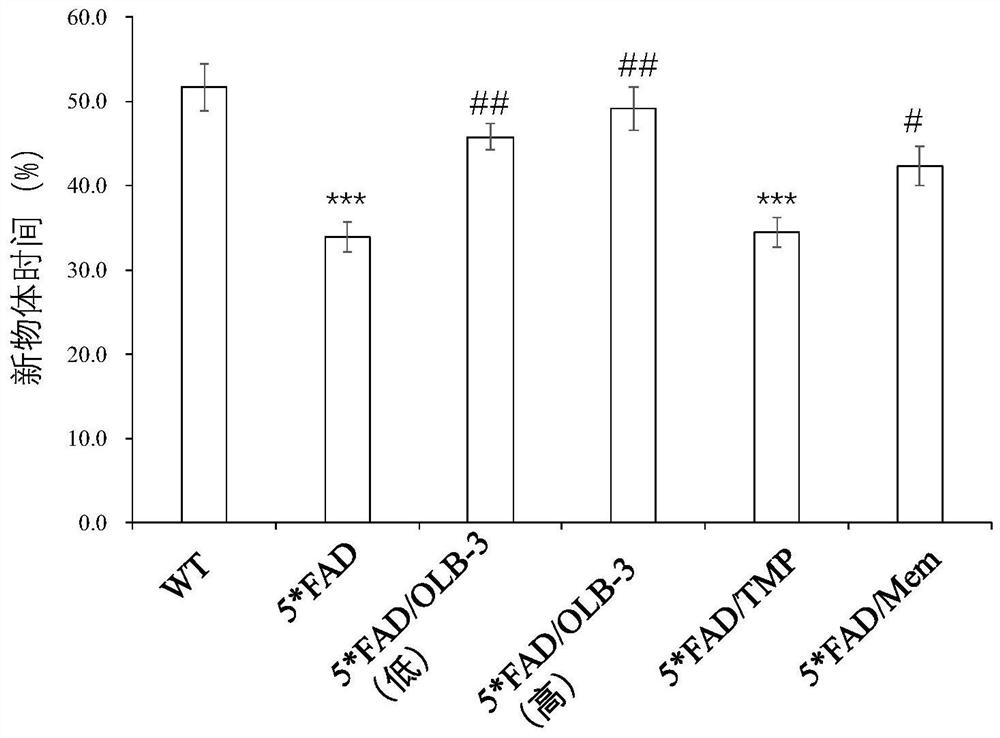
Examples
Embodiment 1
[0057] Synthesis of Compound OLB-3
[0058]
[0059] Ligustrazine (13.6g, 100.0mmol) was dissolved in water (300mL), potassium permanganate (31.6g, 200.0mmol) was added in batches, and stirred at 50°C for 10 hours. After the reaction, cool, adjust the pH to 3 with hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter, and concentrate to obtain the product TMA (10.3 g, 62%). 1 H NMR (400MHz, CDCl 3 )δ2.90(s,3H), 2.61(s,3H), 2.56(s,3H). MS (ESI) m / z: 167.0 [M+H]+.
[0060]
[0061] Compound imidazole (6.2g, 90.5mmol) and tert-butyldimethylsilyl chloride (13.6g, 90.5mmol) were dissolved in N,N-dimethylformamide (200mL), and compound 1a (5.0g, 36.2 mmol), stirred overnight at room temperature. After the reaction, dilute with water, extract with n-hexane, dry over anhydrous sodium sulfate, filter, and concentrate. A part (3.7 g) of the obtained crude product is dissolved in methanol (40 mL), and iodine (0.4 g) is added and stirred for 2 ...
Embodiment 2
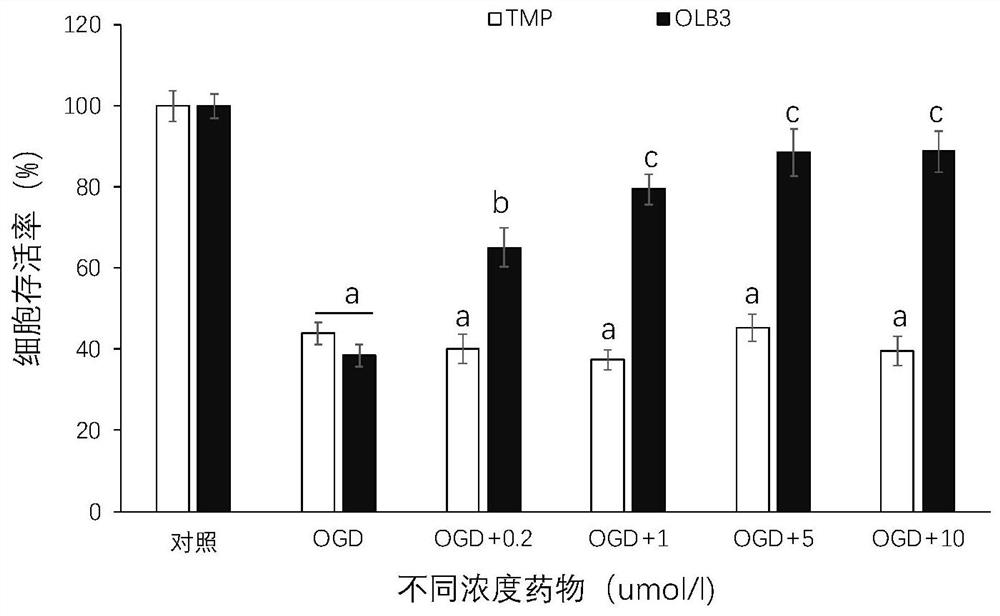
[0068] Example 2: OLB-3 significantly reduces SH-SY5Y cell death caused by OGD
[0069] MTT method was used to detect and evaluate the neuroprotective effect of TMP (tetramethylpyrazine) and its derivatives. Cultivate cells, collect logarithmic phase cells, adjust the concentration of cell suspension, add drug treatment and OGD4h incubation, add MTT-containing culture medium, and incubate After 4 hours, carefully suck out the culture medium in the wells, add 150ulDMSO (dimethyl sulfoxide) to each well, shake at a low speed on a shaker for 10min, so that the crystals are fully dissolved, and measure each well at the OD (absorbance) value of 490nm in an enzyme-linked immunosorbent detector. The absorbance value of the well (set the zeroing well (medium, MTT, dimethyl sulfoxide) at the same time, the control well (cells, the same concentration of drug dissolution medium, culture medium, MTT, dimethyl sulfoxide)). Data representation Mean ± SEM; each group n = 8. One-way analysis ...
Embodiment 3
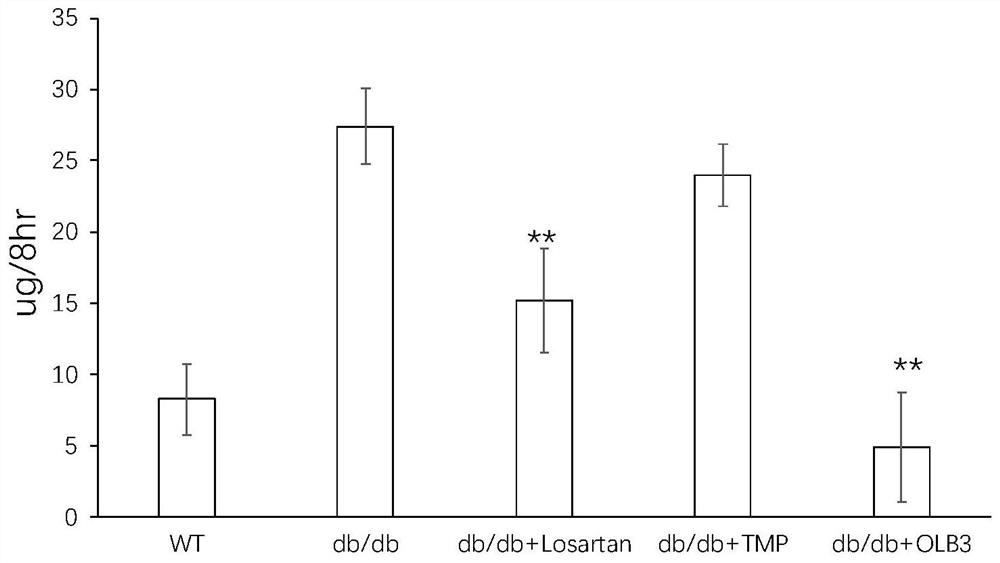
[0071] Example 3: OLB-3 significantly reduces the increase of inflammatory factors and oxidative stress caused by LPS
[0072] The SH-SY5Y cells were revived and cultured, and the cells in the logarithmic growth phase were taken. After 24 hours of culture, the SH-SY5Y neuroblastoma cells were treated with 1 μM all-trans retinoic acid to induce differentiation, and then inoculated into 6-well culture dishes for 24 hours. . Add 0.2uM (L) or 1uM (H) and 1μg / mL LPS to the culture solution for 24 hours, absorb the supernatant culture solution, and measure the changes of inflammatory factors and oxidative stress-related proteins with ELISA kits. The data are expressed as average Value ± SEM; each group n = 8. One-way analysis of variance and multiple comparisons showed that there were differences between the two groups. a, p<0.05vs. LPS group; b, p<0.01vs LPS group; c, p<0.001vs .LPS group.
[0073] Table 1
[0074] (pg / ml) WT LPS LPS+TMP(L) LPS+TMP(H) LPS+OLB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com