Codon-optimized blood coagulation factor VIII gene and construct comprising codon-optimized blood coagulation factor VIII gene
A codon optimization and coagulation factor technology, applied in the field of coagulation factor VIII gene and its constructs, can solve problems such as affecting gene transcription efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1 coagulation factor VIII gene expression framework design
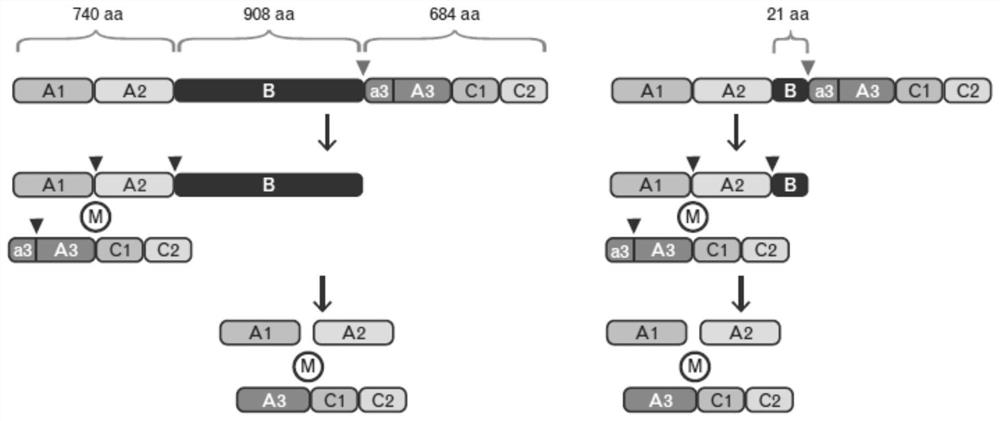
[0074] Based on the amino acid sequence of recombinant human coagulation factor VIII for injection (Ren Jie Xyntha) produced by Pfizer Pharmaceuticals, the expression framework was designed as the wild-type expression framework. The sequence mainly includes A1, A2, B, a3, A3, C1, and C2 regions , in which 887 amino acids are deleted after the B domain is truncated, and only 21 amino acids are contained, and the expression frame number is P0001.
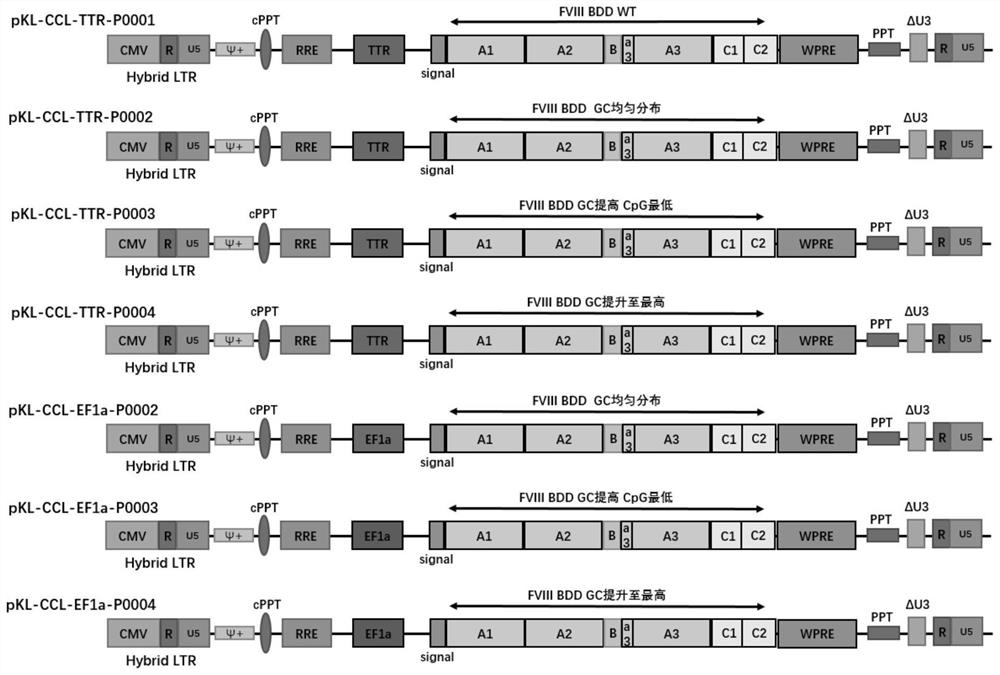
[0075] On the basis of the expression framework P0001, three other expression frameworks P0002, P0003, and P0004 were designed to optimize codons for different conditions. The optimization principle is to preferentially select mammalian cell-biased codons, while avoiding hidden splicing sites, splicing donor and acceptor sequences, immature tailing signals, strong mRNA secondary structures, RNA unstable sequences, Transcription termination signal, etc.
...
Embodiment 2
[0079] Example 2 Construction of coagulation factor VIII gene expression lentiviral vector
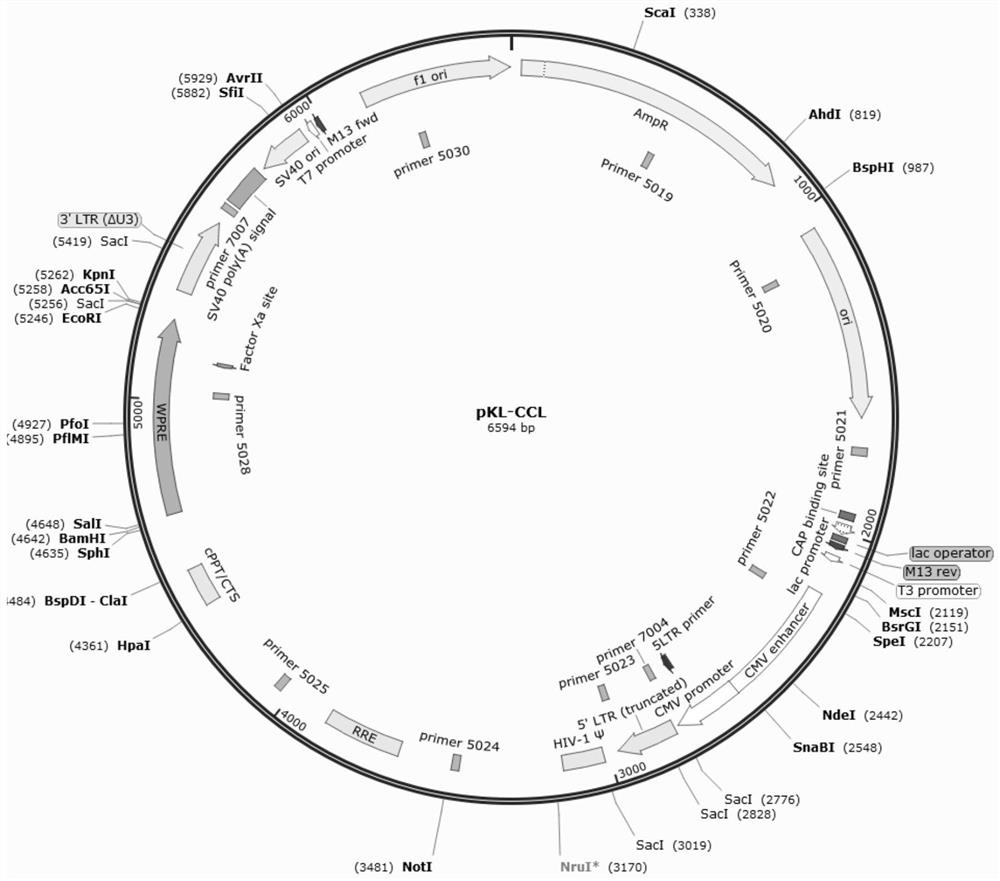
[0080] The coagulation factor VIII gene expression framework P0001 designed in Example 1 was driven by the TTR promoter, and the other expression frameworks were respectively driven by the TTR and EF1a promoters, and then cloned into the lentiviral backbone to form a lentiviral vector. The lentiviral backbone was derived from Kanglin Biotechnology (Hangzhou) Co., Ltd.'s own HIV-1-based third-generation replication-deficient self-inactivating pseudo-enveloped lentiviral backbone—pKL-CCL, nucleotide sequence SEQ ID NO:01, Atlas to show opinions image 3 . The lentiviral backbone contains chimeric LTR promoter, HIV-1 packaging signal (ψ), central polypurine region (cPPT), Rev response element (RRE), polypurine fragment (PPT), woodchuck hepatitis B virus post-transcriptional Regulatory element (WPRE), SV40 virus polyadenylation signal (SV40pA signal), SV40 virus replication initiation si...
Embodiment 3
[0105] Example 3: Coagulation factor VIII gene expression lentiviral packaging
[0106] The coagulation factor VIII gene lentiviral expression vector (pKL-CCL-TTR-P0001, pKL-CCL-TTR-P0002, pKL-CCL-TTR-P0003, pKL-CCL-TTR-P0004, pKL-CCL -EF1a-P0002, pKL-CCL-EF1a-P0003, pKL-CCL-EF1a-P0004), envelope plasmid (pKL-Kan-Vsvg, its nucleotide sequence is shown in SEQ ID NO: 17) and packaging plasmid ( pKL-Kan-Rev, whose nucleotide sequence is shown in SEQ ID NO: 18, pKL-Kan-GagPol, whose nucleotide sequence is shown in SEQ ID NO: 19) co-transfected 293T cells (purchased from the U.S. Type strain collection center (ATCC), preservation number is CRL-3216), in the 293T cell line, carry out the packaging of the coagulation factor VIII gene therapy lentiviral vector. The transfection method is the transient transfection of eukaryotic cells mediated by PEI cationic polymer, the PEI cationic polymer is the PEI-Max transfection reagent purchased from Polysciences (purchased from Polysciences,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com