Composite catalyst as well as preparation method and application thereof
A composite catalyst and catalyst technology, applied in the direction of carbon monoxide or formate reaction preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of low carbonylation efficiency and difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
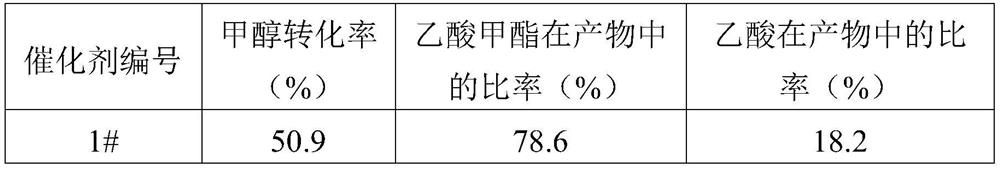
[0112] Weigh 21.46g Zr(NO 3 ) 4 ·5H 2 O, 11.90g Zn(NO 3 ) 2 ·6H 2 O and 7.5g Al(NO 3 ) 3 9H 2 O in a beaker, add 150mL deionized water, stir to obtain salt solution A. Weigh 23.55 g of ammonium carbonate into a beaker, add 150 mL of deionized water, and stir thoroughly to obtain precipitant alkali solution B. Under the condition of vigorous stirring (stirring rate is 450rpm / min), the salt solution A and the precipitant alkali solution B are mixed in a parallel flow mode, and the relative flow rate of the solutions A and B is adjusted to ensure that the pH of the precipitation mixture is kept between 7 and 8 between. After co-precipitation, aging for 2h. Afterwards, it was dried in an oven at 100°C for 6 hours, and then calcined in a muffle furnace at 500°C for 4 hours to obtain a water vapor shift catalyst. According to XRF elemental analysis, the composition of the water vapor shift catalyst is (ZnO) 0.4 (ZrO 2 ) 0.5 (Al 2 o 3 ) 0.1 .
[0113] H-MOR (Si / Al=1...
Embodiment 2
[0119] The same preparation method and preparation conditions as in Example 1 were used to obtain a water vapor shift catalyst. The specific preparation conditions of the modified H-MOR molecular sieve are shown in Table 2 below, and the rest of the operations are the same as in Example 1. The method and conditions for preparing the composite catalyst using the water vapor catalyst and the modified H-MOR molecular sieve are the same as in Example 1.
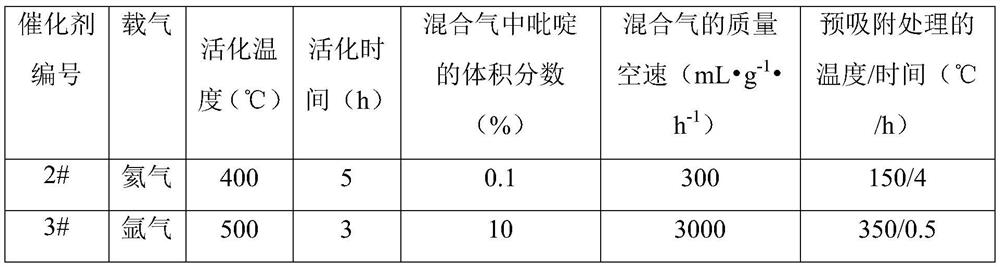
[0120] Table 2
[0121]
[0122] Catalyst 2#: The difference from Example 1 is that the carrier gas is helium during the pre-adsorption of pyridine by the H-MOR molecular sieve.
[0123] Catalyst 3#: The difference from Example 1 is that the carrier gas is helium during the pre-adsorption of pyridine by the H-MOR molecular sieve.
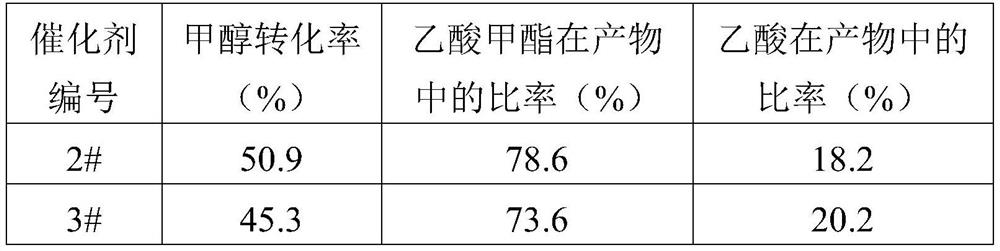
[0124] 2# and 3# catalysts are evaluated, the reaction conditions are consistent with Example 1, and the reaction evaluation results are shown in Table 3.
[0125] Table 3 embodiment 2 catalyst r...
Embodiment 3
[0129] Co-precipitation method is used to prepare water vapor shift catalysts with different metal compositions and different contents, wherein the composition of water vapor shift catalysts is different from that of Example 1 and Example 3. The rest of the operations and conditions of the coprecipitation method are the same as those of Example 1, and the rest of the operations and conditions of the impregnation method are the same. Condition is the same as embodiment 3. The obtained catalysts are respectively recorded as 5# to 9#, and the specific composition of each catalyst is shown in Table 4. Catalysts 5# to 9# were evaluated under the same reaction conditions as in Example 1, and the reaction products were analyzed online by gas chromatography. The analysis results are shown in Table 4.
[0130] Table 4 embodiment 3 catalyst reaction result
[0131]
[0132] The composition of the water vapor shift catalyst samples was determined by XRF.
[0133] Table 4 shows that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com