Antibacterial absorbable medical soft tissue suture line and preparation method and application thereof
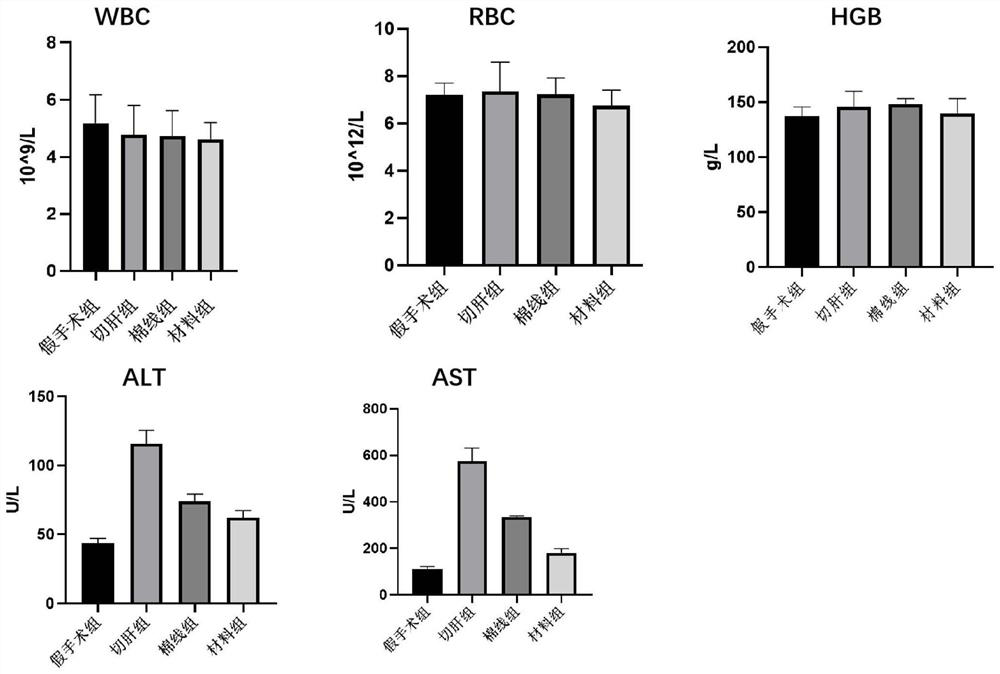
A suture and soft tissue technology, applied in the field of medical suture, can solve the problems of insufficient mechanical strength, unfavorable large-scale application, easy to lose water and difficult to place and store, achieve excellent adaptability, reduce secondary injuries, and avoid secondary damage. The effect of secondary damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A preparation method of antibacterial absorbable suture, comprising the following steps:
[0053] (1) 2.5g chitosan (molecular weight 1000) is dissolved in 40ml dimethyl sulfoxide (DMSO), under nitrogen protection, add 1.5g p-aldehyde benzoic acid, 10mg 4-dimethylaminopyridine (DMAP), 5 mg of dicyclohexylcarbodiimide, reacted at room temperature 25°C for 24 hours, added water to precipitate, filtered, washed several times with water, dried and dialyzed with a dialysis bag with a molecular weight of 1000 for 3 days to obtain graft-modified aldehydated chitosan ;
[0054] (2) 0.6g carboxymethyl chitosan powder is dissolved in the deionized water of 10ml, 800rpm stirs 4h and dissolves completely and forms light yellow solution;
[0055] (3) Disperse 0.1 g of graft-modified aldehydated chitosan into 5 ml of deionized water, ultrasonically disperse it for 10 min, and slowly add it to the carboxymethyl chitosan solution, then Stir at 800rpm for 2h to a uniform solution to o...
Embodiment 2
[0059] A preparation method of antibacterial absorbable suture, comprising the following steps:
[0060] (1) 2.5g chitosan (molecular weight 1000) is dissolved in 40ml dimethyl sulfoxide (DMSO), under nitrogen protection, add 1.5g p-aldehyde benzoic acid, 10mg 4-dimethylaminopyridine (DMAP), 5 mg of dicyclohexylcarbodiimide, reacted at room temperature 25°C for 24 hours, added water to precipitate, filtered, washed several times with water, dried and dialyzed with a dialysis bag with a molecular weight of 1000 for 3 days to obtain graft-modified aldehydated chitosan ;
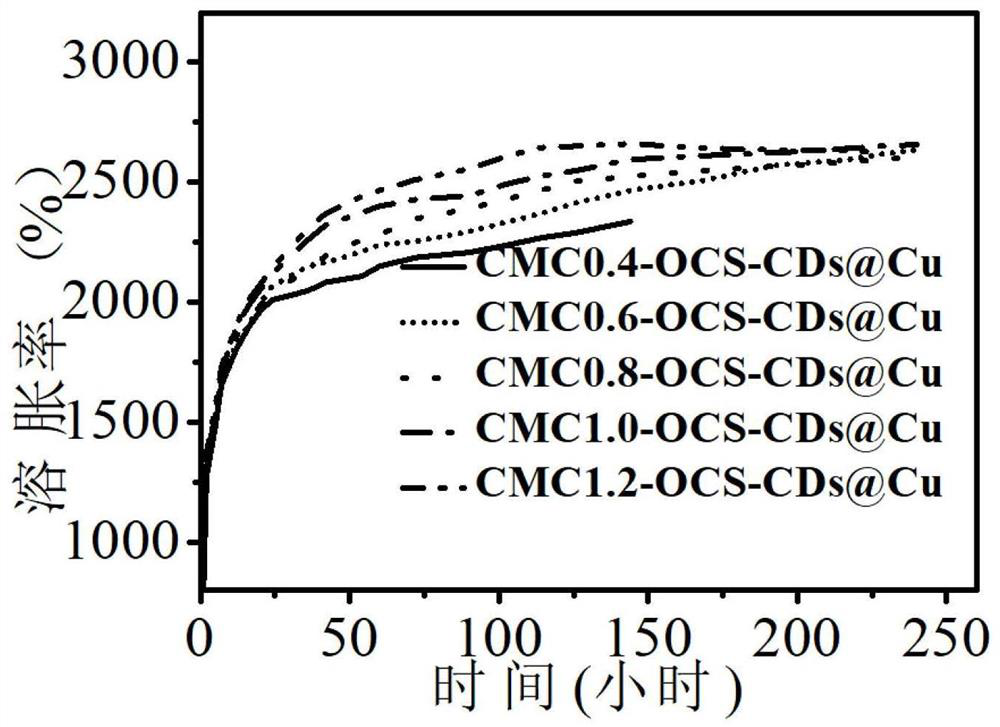
[0061] (2) Dissolve 0.4, 0.6, 0.8, 1.0, 1.2g of carboxymethyl chitosan powder in 10ml of deionized water respectively, and stir at 800rpm for 4h to completely dissolve to form a light yellow solution;
[0062] (3) Disperse 0.1 g of graft-modified aldehydated chitosan into 5 ml of deionized water, ultrasonically disperse it for 10 min, and slowly add it to the carboxymethyl chitosan solution, then Stir at 800r...
Embodiment 3
[0067] A preparation method of antibacterial absorbable suture, comprising the following steps:
[0068] (1) 2.5g chitosan (molecular weight 1000) is dissolved in 40ml dimethyl sulfoxide (DMF), and 1.5g p-aldehyde benzoic acid, 10mg 4-dimethylaminopyridine (DMAP) are added under nitrogen protection, 5 mg of dicyclohexylcarbodiimide, reacted at room temperature 25°C for 24 hours, added water to precipitate, filtered, washed several times with water, dried and dialyzed with a dialysis bag with a molecular weight of 1000 for 3 days to obtain graft-modified aldehydated chitosan ;
[0069] (2) 1.2g carboxymethyl chitosan powder is dissolved in the deionized water of 10ml, 800rpm stirs 4h and dissolves completely and forms light yellow solution;
[0070] (3) Disperse 0.2 g of graft-modified aldehylated chitosan into 5 ml of deionized water, ultrasonically disperse it for 10 min, and slowly add it to the carboxymethyl chitosan solution, then Stir at 800rpm for 2h to a uniform solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com