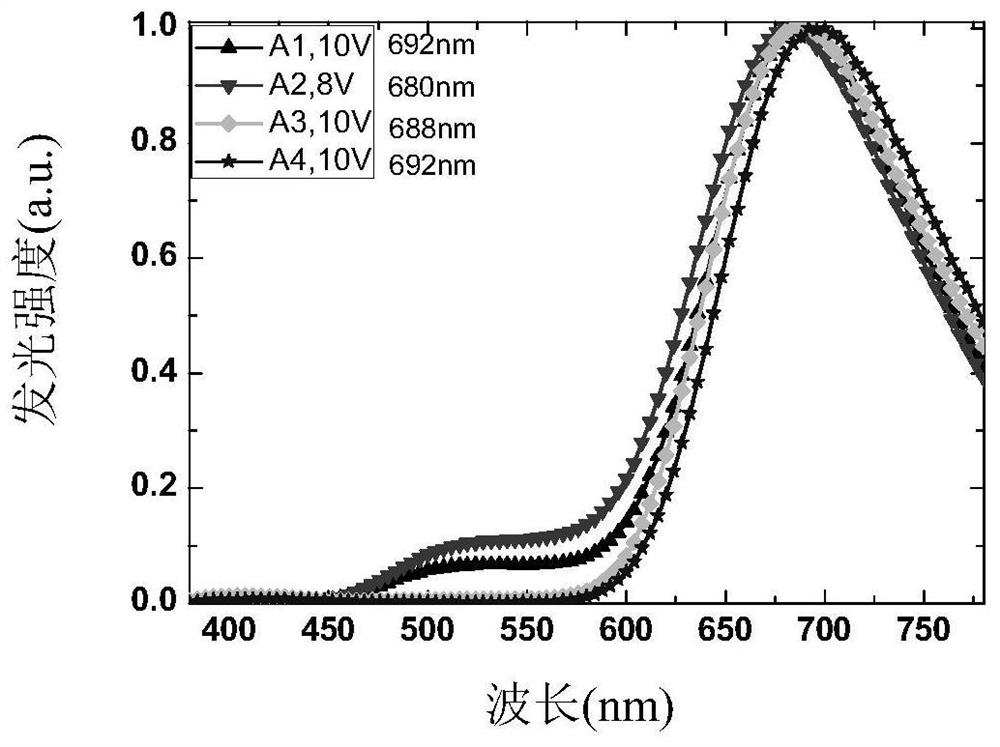
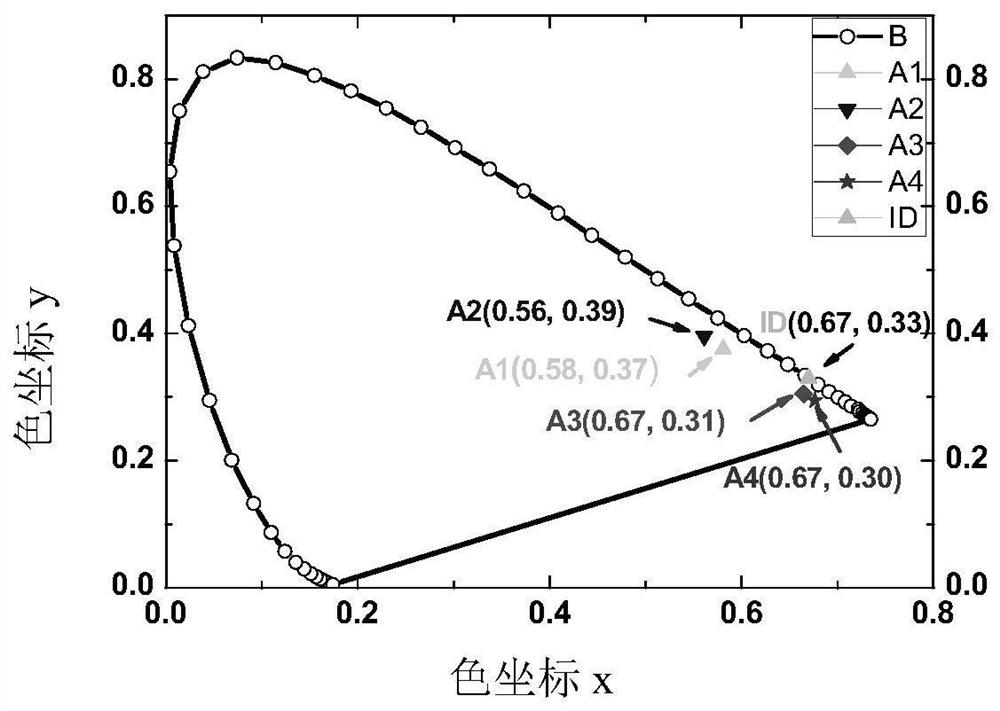
High-efficiency non-doped electro-induced long-wave red light arylamine diphenyl trans-butene dinitrile derivative
A technology of arylamine diphenyl and butenedinitrile, which is applied in the preparation of amino compounds, circuits, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
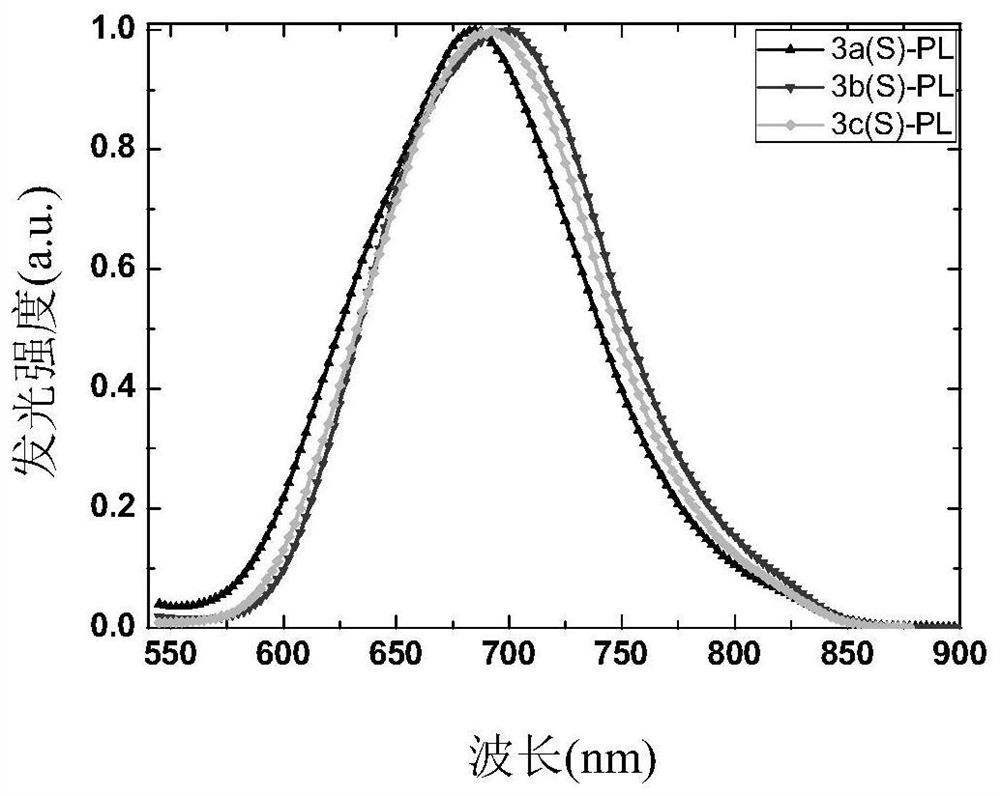
[0060] Example 1 Synthesis of two (4-(N-(4-biphenyl)-9,9-diethyl-2-fluorenamine) phenyl) trans-butenedinitrile (BPFAFN, 3b):
[0061] 1. Preparation of N-(4-biphenyl)-9,9-diethyl-2-fluorenamine (2b)
[0062] Under argon protection, 301mg (1mmol) 2-bromo-9,9-diethylfluorene, 203mg (1.2mmol) 4-benzidine, 134mg (1.4mmol) sodium tert-butyl alkoxide were successively added to a 100mL round bottom flask , 1.9 mg (0.01 mmol) Pd (OAc) 2 , 14.3mg (0.03mmol) organophosphine Xphos, dissolved in 25mL of toluene. Temperature was 110°C, reacted for 20 hours; after cooling, 80 mL of brine was added, extracted with ethyl acetate (2×80 mL, twice, 80 mL each time), after the organic phase was dried and concentrated, column chromatography (silica gel, dichloromethane / petroleum ether eluent) to obtain white solid N-(4-biphenyl)-9,9-diethyl-2-fluorenamine (2b) with a yield of 79.4%.
[0063] Product Confirmation:
[0064] 1 H NMR (400MHz, CDCl 3 )–δ(ppm):0.36-0.39(t,6H),1.92-2.02(m,4H),7.08-...
Embodiment 2
[0071] Example 2 Synthesis of two (4-(N-(2-spirobifluorenyl)-3,5-xylaniline) phenyl) trans-butenedinitriles (SFPAFN, 3a):
[0072] 1. Preparation of N-(3,5-xylyl)-2-spirobifluorenamine (2a)
[0073] Take 395mg (1mmol) of 2-bromo-spirobifluorene and 145mg (1.2mmol) of 3,5-xylidine, and the amount and method of other compounds are the same as step 1 of Example 1 to obtain a white solid (2a) with a yield of 62.4 %.
[0074] Product Confirmation:
[0075] 1 H NMR (400MHz, CDCl 3 )–δ(ppm):2.284(s,6H),6.718–6.737(d,2H),6.753–7.081(m,6H),7.132–7.172(m,2H),7.261–7.322(m,4H), 7.422–7.605(m,4H);
[0076] Elemental analysis (molecular formula C 33 h 25 N): Theoretical: C, 91.00; H, 5.79; N, 3.22. Test values: C, 90.88; H, 5.71; N, 3.18.
[0077] 2. Preparation of bis(4-(N-(2-spirobifluorenyl)-3,5-xylaniline) phenyl) transbutenedinitrile (SFPAFN, 3a)
[0078] Take 958mg (2.2mmol) N-(3,5-xylyl)-2-spirobifluorenamine (2a), and the amount and method of other compounds are the same ...
Embodiment 3
[0082] Example 3 Synthesis of two (4-(N,N-bis(4-biphenyl)amino)phenyl)trans-butenedinitrile (BBPAFN, 3c):
[0083] 1. Preparation of N,N-bis(1-biphenyl)amine (2c)
[0084] Take 233mg (1mmol) of 4-bromobiphenyl and 203mg (1.2mmol) of 4-aminobiphenyl, and the amounts and methods of other compounds are the same as step 1 of Example 1. An off-white solid (2c) was obtained in 67.8% yield.
[0085] Product Confirmation:
[0086] 1 H NMR (400MHz, CDCl 3 )–δ(ppm):7.192–7.273(d,4H),7.461–7.482(m,6H),7.523–7.544(m,4H),7.585–7.596(m,4H);
[0087] Elemental analysis (molecular formula C 24 h 19 N): Theoretical: C, 89.68; H, 5.96; N, 4.36. Test values: C, 89.56; H, 5.92; N, 4.28.
[0088] 2. Preparation of bis(4-(N,N-bis(4-biphenyl)amino)phenyl)trans-butenedinitrile (BBPAFN, 3c)
[0089] Take 706 mg (2.2 mmol) of N,N-bis(1-biphenyl)amine (2c), and the amount and method of other compounds are the same as Step 2 of Example 1 to obtain a red solid (3c), with a yield of 68.6%.
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com